We have seen that molar mass and molecular structure influence the boiling point of a substance. We
Question:
We have seen that molar mass and molecular structure influence the boiling point of a substance. We can see these two factors at work in the boiling points of the group 6A hydrides shown in the following graph.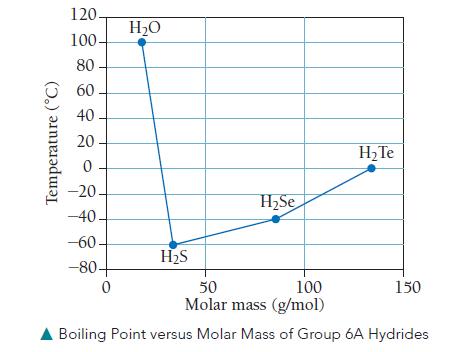
In order to disentangle the effects of molar mass and molecular structure on the boiling point, consider the data in the following table.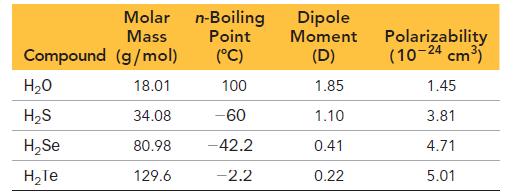
Use the information in the graph and the table to answer the following questions.
a. Does molar mass alone correlate with the trend in the boiling points for the group 6A hydrides?
b. Which boiling points in the graph correlate with polarizability?
What type of intermolecular force correlates with polarizability?
c. Use the data in the table to explain the anomalously high boiling point of water.
Step by Step Answer:






