Question: Write complete ionic and net ionic equations for each reaction. (a) 3 SrCl (aq) + 2 Li3PO4(aq) + KOH(aq) (b) HCH3O2(aq) Sr3 (PO4)2(s) +6 LiCl(aq)
Write complete ionic and net ionic equations for each reaction.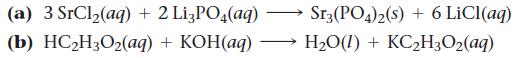
(a) 3 SrCl (aq) + 2 Li3PO4(aq) + KOH(aq) (b) HCH3O2(aq) Sr3 (PO4)2(s) +6 LiCl(aq) HO(1) + KCH3O(aq)
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
a Write the complete ionic equation by separating strong electrolytes into ... View full answer

Get step-by-step solutions from verified subject matter experts


