Acetic acid, CH 3 CO 2 H, is made industrially by the reaction of methanol and carbon
Question:
Acetic acid, CH3CO2H, is made industrially by the reaction of methanol and carbon monoxide.
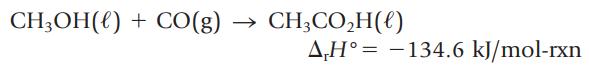
What is the enthalpy change for producing 1.00 L of acetic acid (d = 1.044 g/mL) by this reaction?
Transcribed Image Text:
CH₂OH() + CO(g) CH3CO₂H() AH° -134.6 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
enthalpy change for producing 100 L of acetic acid CH3CO2H by the given reaction you need to use the ...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
Ammonia gas is made industrially by the Haber process, which involves the reaction between the gases nitrogen and hydrogen. The amount of ammonia gas produced from this reaction is affected by both...
-
Tony acquired 1,000 shares in X Co (a resident public company) for $10 each in August 2000. In January this year X Co returned $7 of capital to its shareholder in respect to each share they held. The...
-
Using the information in Problem 1, determine the sample size needed if the standard time estimate is to be within 5 percent of the true mean 99 percent of the time.
-
A us corporation has two foreign marketing branches, one in France and one in Hong Kong. The current situation is summarized as follows (all numbers in thousands of usd): (a) The us tax rate is 30...
-
Determine the \(x\)-component of the acceleration, \(a_{x}\), along the centerline \((y=0)\) for the flow of Problem 4.20. Can you determine the acceleration vector at a location not on the...
-
At January 1, 2016, Hilly Mountain Flagpoles had Accounts Receivable of $31,000, and Allowance for Bad Debts had a credit balance of $3,000. During the year, Hilly Mountain Flagpoles recorded the...
-
Critically analyze the following question. PROVIDE REFERENCES What job factors are most important in the workplace of today?
-
Assume you mix 100.0 mL of 0.200 M CsOH with 50.0 mL of 0.400 M HCl in a coffee-cup calorimeter. The following reaction occurs: The temperature of both solutions before mixing was 22.50C, and it...
-
Isooctane (2,2,4-trimethylpentane), one of the many hydrocarbons that make up gasoline, burns in air to give water and carbon dioxide. What is the enthalpy change if you burn 1.00 L of isooctane (d =...
-
Define and explain the key components of a prevention protocol and a recovery protocol.
-
As of april 3 0 , $ 1 , 6 6 7 of interest expense has accured on a note payable. The full intrest payment of $ 5 0 0 0 on the note is due may 2 0 . Record the payment of interest for may 2 0 th .
-
What method is being used when someone attempts to measure inflation expectations by going door-to-door asking people questions on what they expect and by sending out fliers for people to fill out?
-
Creative Computing sells a tablet computer called the Protab. The $ 7 8 0 sales price of a Protab Package includes the following: Required: 1 . & 2 . Indicate below whether each item is a separate...
-
What is the ex - dividend present value of a firm's current and future earnings if the interest rate is 7 % , the expected growth rate of the firm is 4 % , and the firm's current profits are $ 7 5...
-
Suppose a household has a total income of $60,000 and pays $15,000 in taxes. If the MPC is 0.8, then how much is the household's total consumption? Suppose a household has a total income of $60,000...
-
How is the predetermined overhead rate used to allocate overhead?
-
Give codons for the following amino acids: (a) Th (b) Asp (c) Thr
-
Count the total number of s bonds and p bonds in the compound below: -3-N
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
Use the tabulated values of the enthalpy of combustion of benzene and the enthalpies of formation of CO 2 (g) and H 2 O(l) to determine H o f for benzene.
-
After 5 years, Mike\'s account earned $ 9 0 0 in interest. If the interest rate ( in decimal form ) is 0 . 1 5 , how much did Mike initially invest?
-
Nancy is a 50% partner in the PM Partnership and has an outside basis of $112,000 at the end of the year prior to any distributions. On December 31, Nancy receives a proportionate operating...
-
2. Let f(x)=-3x+11. +11. Write an equation for g(x), the inverse of f.

Study smarter with the SolutionInn App


