Isooctane (2,2,4-trimethylpentane), one of the many hydrocarbons that make up gasoline, burns in air to give water
Question:
Isooctane (2,2,4-trimethylpentane), one of the many hydrocarbons that make up gasoline, burns in air to give water and carbon dioxide.
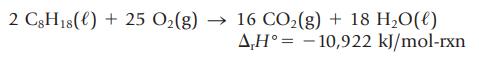
What is the enthalpy change if you burn 1.00 L of isooctane (d = 0.69 g/mL)?
Transcribed Image Text:
2 C8H18 () + 25 O₂(g) → 16 CO₂(g) + 18 H₂O(l) A,H° -10,922 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To calculate the enthalpy change when burning 100 L of isooctane 224trimethylpentane you first need ...View the full answer

Answered By

Ashish Bhalla
I have 12 years work experience as Professor for Accounting, Finance and Business related subjects also working as Online Tutor from last 8 years with highly decentralized organizations. I had obtained a B.Com, M.Com, MBA (Finance & Marketing). My research interest areas are Banking Problem & Investment Management. I am highly articulate and effective communicator with excellent team-building and interpersonal skills; work well with individuals at all levels.
4.80+
17+ Reviews
46+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Butane (C 4 H 10 ) burns in air to give carbon dioxide and water.
-
Solid oxide fuel cells (SOFC) have been proposed as an alternative energy technology for use in large stationary power applications (1 to 10MWof electrical power). These devices have an ion...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Scandinavo Ltd. is a CCPC that began operations on January 1, 2020 when it was first incorporated and a calendar fiscal period was chosen. Scandinavo Ltd. Is not associated with any other...
-
Explain the difference between time-based and output-based compensation plans.
-
What are the main differences between an independent agent and a dependent agent? A dependent agent and a branch? A branch and a subsidiary? A subsidiary and a joint venture?
-
A certain flow field has the velocity vector \(\mathbf{V}=\frac{-2 x y z}{\left(x^{2}+y^{2} ight)^{2}} \mathbf{i}+\frac{\left(x^{2}-y^{2} ight) z}{\left(x^{2}+y^{2} ight)^{2}}...
-
In each of the following, identify which of the elements of the fundamental principles is most applicable. In addition, discuss what action(s) (if any) you believe auditors should take with respect...
-
Captain Jack Sparrow has been marooned on an island in the Atlantic by his crew, and decides to build a raft to escape. The wind seems quite steady, and first blows him due east for 11 km, and then...
-
Acetic acid, CH 3 CO 2 H, is made industrially by the reaction of methanol and carbon monoxide. What is the enthalpy change for producing 1.00 L of acetic acid (d = 1.044 g/mL) by this reaction?...
-
Calcium carbide, CaC 2 , is manufactured by the reaction of CaO with carbon at a high temperature. (Calcium carbide is then used to make acetylene.) Is this reaction endothermic or exothermic? What...
-
On the basis of the following stock information, describe the features of the stock and assess its performance: dividends per share = $0.80, current share price = $28.50, current dividend yield = 2.8...
-
In each of the following, the starting price is $ 7 5 and the ending price is either $ 1 0 0 or $ 5 0 for a change of $ 2 5 . What is the percentage return for each transaction? ( This "holding...
-
Local Governments (use Miami-Dade County) - How and when was the current structure created; what does the current structure consist of; who are the current individuals involved; what is the...
-
Talha scores 1 9 goals in Malaysian Super league in 2 0 1 1 season. If his scoring capabilities increase by 1 5 % every year, what would it his score in the following 5 years?
-
Shula Company had the following transactions in Year 3 : July 3 1 Sold a delivery truck for $ 2 5 , 0 0 0 cash. The delivery truck originally cost $ 5 7 , 0 0 0 and had accumulated depreciation of $...
-
find the service revenue On August 1, 2025, the following were the account balances of Monty Repair Services. Cash Accounts Receivable Notes Receivable Supplies Equipment Debit $5,560 Accumulated...
-
What is the formula to compute the predetermined overhead allocation rate?
-
Quadrilateral EFGH is a kite. Find mG. E H <105 G 50 F
-
For each pair of compounds below, predict which compound will have the higher boiling point and explain your choice: a) CH 3 CH 2 CH 2 OCH 3 or CH 3 CH 2 CH 2 CH 2 OH b) CH 3 CH 2 CH 2 CH 3 or CH 3...
-
Derive the following expression for calculating the isothermal change in the constant volume heat capacity: (CV/V)T = T (2P/T2)V.
-
For each of the following compounds, identify all groups that would be considered substituents, and then indicate the systematic name as well as the common name for each substituent: (a) (b) (c) (d)...
-
Gentry Wholesalers accepts from Marigold Stores a $9,780, 4-month, 10% note dated May 31 in settlement of Marigold's overdue account. The maturity date of the note is September 30. What entry does...
-
On January 1, 2022,Shot Co. acquired 75% of Good Inc Information on acquisition date: Good tidentifiable assets have a carrying amount of Php70,000 and fair value Php100,000. The difference is due to...
-
Ronaldo and Liu, Co. reported the following information for the year ended December 31, 2019 Accounts payable increased during the year by $15,000; Inventory decreased during the year by $14,000;...

Study smarter with the SolutionInn App


