The formation of NO from N 2 and O 2 is unfavorable at 298 K, but it
Question:
The formation of NO from N2 and O2 is unfavorable at 298 K, but it becomes increasingly favored at high temperatures such as those in an automobile cylinder. Using data (ΔfH°, S° and ΔfG°) in Appendix L, calculate the equilibrium constant for the reaction ½ N2(g) + ½ O2(g) → NO(g) at 298 K and at 1000 K.
Data given in Appendix L
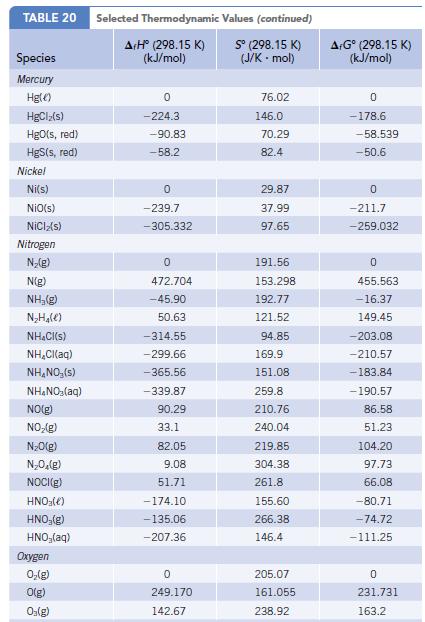
Transcribed Image Text:
TABLE 20 Species Mercury Hg() HgClz(s) HgO(s, red) HgS(s, red) Nickel Ni(s) NiO(s) NICIz(s) Nitrogen N₂(g) N(g) NH,(g) N₂H₂(e) NH₂Cl(s) NH₂Cl(aq) NH₂NO, (s) NH4NO₂(aq) NO(g) NO₂(g) N₂O(g) N₂O₂(g) NOCI(g) HNO3(e) HNO₂(g) HNO₂(aq) Oxygen 0₂(g) O(g) O₂(g) Selected Thermodynamic A+Hº (298.15 K) (kJ/mol) 0 -224.3 -90.83 -58.2 0 -239.7 -305.332 0 472.704 -45.90 50.63 -314.55 -299.66 -365.56 -339.87 90.29 33.1 82.05 9.08 51.71 -174.10 -135.06 -207.36 0 249.170 142.67 Values (continued) Sº (298.15 K) (J/K-mol) 76.02 146.0 70.29 82.4 29.87 37.99 97.65 191.56 153.298 192.77 121.52 94.85 169.9 151.08 259.8 210.76 240.04 219.85 304.38 261.8 155.60 266.38 146.4 205.07 161.055 238.92 A+Gº (298.15 K) (kJ/mol) 0 -178.6. -58.539 -50.6 0 -211.7 -259.032 0 455.563 -16.37 149.45 -203.08 -210.57 -183.84 -190.57 86.58 51.23 104.20 97.73 66.08 -80.71 -74.72 -111.25 0 231.731 163.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Formation of NO from N2 and O2 The formation of NO from N2 and O2 is an endothermic reaction meaning ...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Explain, using Le Chtelier's principle, why the equilibrium constant for the formation of NO from N2 and O2 increases with increasing temperature, whereas the equilibrium constant for the formation...
-
The equilibrium constant (KP) for the formation of the air pollutant nitric oxide (NO) in an automobile engine at 530°C is 2.9 Ã 10-11: (a) Calculate the partial pressure of NO under these...
-
Write sentences for the long-term direction and strategic path that management intends to follow. "Where we are headed?" and should explain why the direction in which you intend to point the company...
-
Use the following information for this question: Taxable income Marginal tax rate 15% 25% 34% 39% 34% 35% S S 0-S 50,000 75,000 50,000-$ S 75,000 $100,000 $ 100,000-S 335,000 S 335,000-$10,000,000...
-
IBM recently saved $250 million over three years by implementing supply chain software that reduced the cost of components used in its manufacture of computers. If we assume that the savings occurred...
-
Identify the five main types of ratios used in this chapter to analyze a company. What does each group of ratios attempt to measure?
-
The table shows the results of a survey in which 300 managers and 300 executives aged 25 to 50 were asked if they go for annual leisure trips. (a) Find the probability that a randomly selected...
-
On January 1, 2017, Nicks Corporation issued $250 million of floating-rate debt. The debt carries a contractual interest rate of "LIBOR plus 5.5%," which is reset annually on January 1of each year....
-
What is the Profit maximizing product level and why? What is the Profit maximizing price and why? *This is for Q4, the 50 and $60 is incorrect Total Profits/ Price Output TR MR TC TFC TVC AFC AVC ATC...
-
Acetonitrile, CH 3 CN, is an important solvent. The chemical is normally available as a by-product of the manufacture of acrylonitrile, CH 2 =CHCN, the building block of polyacrylonitrile, a widely...
-
Chlorine atoms are formed by photochemical reactions of chlorofluorocarbons in the upper atmosphere. Using the average bond energy of the CCl bond in Table 8.8, calculate the wavelength of radiation...
-
A large crystal is constructed by stacking small, identical pieces of crystal, much as you construct a brick wall by stacking bricks. A unit cell is the smallest such piece from which a crystal can...
-
Write a letter stating why do you want to be a Zeta Phi Beta? And why did you choose that sorority?
-
What happens if there's something wrong on a person's credit report? Do applicants have a way of checking that information before applying for a job? Explain.
-
question Background: On 30July2022, Prime Minister Anthony Albanese announceddraft words for a constitutional amendment on an Aboriginal and Torres Strait Islander Voice. He also proposed a draft...
-
Explain the following thoroughly for recitation guide purposes. 1. Distinction between Administrative Law and International Law ; Administrative Law versus Constitutional Law; Administrative Law vs...
-
Topic: Design and develop a report on cross cultural communication and management. Assume you are a US manager of a subsidiary in Japan and explain how differences on Hofstede's four dimensions of...
-
Movie studios segment their markets by age. Two segments that are particularly important to this industry are teenagers and 20- to 30-year-olds. To assess markets and guide the making of movies, a...
-
Cobb Manufacturing Company uses a process cost system and average costing. The following production data is for the month of June 2011. Production Costs Work in process, beginning of the month:...
-
Consider the following circuit where the combinational circuit is represented by COMB and clock skew is represented by t skew . Given the following parameters: FF setup time = 20 ns FF hold time = 10...
-
Consider the following circuit where the combinational circuit is represented by COMB and clock skew is represented by tskew. Given the following parameters: FF setup time = 10 ns FF hold time = 2 ns...
-
A Mealy sequential machine has the following state table: Complete the following timing diagram. Clearly mark on the diagram the times at which you should read the values of Z. All state changes...
-
A corporate bond with a $1,000 face value pays a $50 coupon semi-annually. The bond will mature in ten years, and has a nominal yield to maturity of 9 percent. What is the price of the bond?
-
A copper kettle weighs approximately 0.98 kg. Suppose this kettle contains 800 mL of water at a temperature of 21.5C. How much heat (in kJ) would be required to raise the temperature of the water to...
-
Prepare the current assets section of the balance sheet at December 31 for Bin Manufacturing using the following Information. Hint. Not all Information given is needed for the solution. Cash Accounts...

Study smarter with the SolutionInn App


