Use standard enthalpies of formation in Appendix L to calculate enthalpy changes for the following: (a) 0.054
Question:
Use standard enthalpies of formation in Appendix L to calculate enthalpy changes for the following:
(a) 0.054 g of sulfur burns, forming SO2(g)
(b) 0.20 mol of HgO(s) decomposes to Hg(ℓ) and O2(g)
(c) 2.40 g of NH3(g) is formed from N2(g) and excess H2(g)
(d) 1.05 × 10−2 mol of carbon is oxidized to CO2(g)
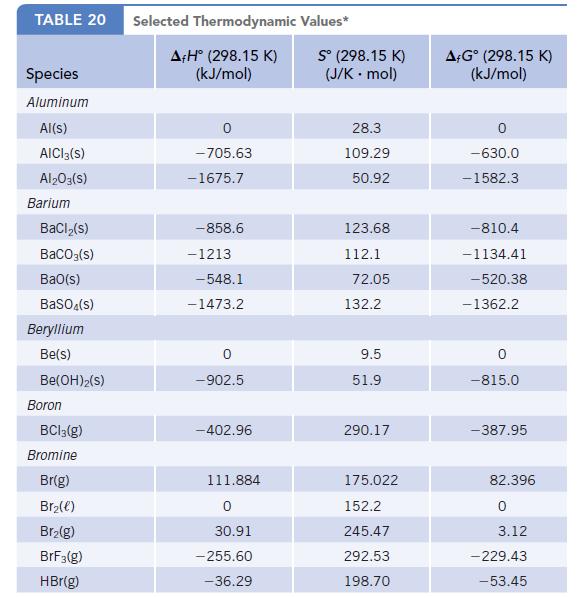
Data given in Appendix L
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





