Use the standard reduction potentials (Appendix M) for the half-reactions Hg 2 Cl 2 (s) + 2e
Question:
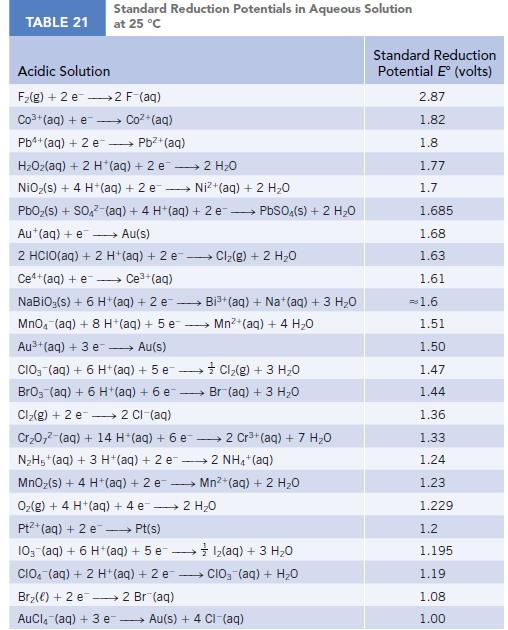
Use the standard reduction potentials (Appendix M) for the half-reactions Hg2Cl2(s) + 2e− → 2 Hg(ℓ) + 2 Cl−(aq) and Hg22+(aq) + 2 e− → 2 Hg(ℓ) to calculate the value of Ksp for Hg2Cl2.
Data given in Appendix M
Transcribed Image Text:
TABLE 21 Standard Reduction Potentials in Aqueous Solution at 25 °C Acidic Solution F₂(g) + 2 e 2 F-(aq) Co3+(aq) + e Coz+(aq) Pb4+ (aq) + 2 e - Pb²+ (aq) HzOz(aq) + 2 H*(aq) +2e → 2H2O NiO₂(s) + 4 H+ (aq) + 2 e→→→→→→ Ni+(aq) + 2 HO PbO₂ (s) + SO4² (aq) + 4 H+ (aq) + 2e → PbSO4(s) + 2 H₂O Au+ (aq) + e→→→→→ Au(s) 2 HCIO(aq) + 2 H+ (aq) + 2 e-- - Ce+(aq) + e→→→→ Ce³+ (aq) NaBiO;(s) + 6 H+ (aq) + 2 e- → MnO4 (aq) + 8 H+(aq) + 5 e Au³+ (aq) + 3 e→→→→→ Au(s) CIO3(aq) + 6 H+ (aq) + 5 e→→→→→→ BrO3 (aq) + 6 H+ (aq) + 6 e- Cl₂(g) + 2 e 2 Cl-(aq) Cr₂0,² (aq) + 14 H*(aq) + 6 e 2 Cr³+ (aq) + 7 H₂O N₂H5+ (aq) + 3 H+ (aq) + 2 e2 NH4+ (aq) MnO₂(s) + 4 H + (aq) + 2 e O₂(g) + 4 H+ (aq) + 4 e 2 H₂O Pt+ (aq) + 2 e →→→→→ Pt(s) 10- (aq) + 6 H+ (aq) + 5 e1₂(aq) + 3 H₂O CIO (aq) + 2 H+ (aq) + 2 e CIO₂ (aq) + H₂O Br₂() +2 e 2 Br(aq) AuCl(aq) + 3 e → Cl₂(g) + 2 H₂O → Bi³+ (aq) + Na+ (aq) + 3 H₂O Mn²+ (aq) + 4 H₂0 Cl₂(g) + 3 H₂O Br (aq) + 3 H₂O Mn²+ (aq) + 2 H₂O Au(s) + 4 CI-(aq) Standard Reduction Potential E (volts) 2.87 1.82 1.8 1.77 1.7 1.685 1.68 1.63 1.61 = 1.6 1.51 1.50 1.47 1.44 1.36 1.33 1.24 1.23 1.229 1.2 1.195 1.19 1.08 1.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
Hg2Cl2 2e 2 Hg 0 2 Claq Hg2aq 2e 2 Hg0 E 027 v E0789 v Ecell E oxidation ...View the full answer

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Determine the reactions at the beam supports for the given loading. 50 lb/in 400 lb 12 in 20 in 6 in
-
Use the standard reduction potentials (Appendix M) for the half-reactions [AuCl 4 ] (aq) + 3 e Au(s) + 4 Cl (aq) and Au 3+ (aq) + 3 e Au(s) to calculate the value of K formation for the complex...
-
Use the standard reduction potentials (Appendix M) for the half-reactions [Zn(OH) 4 ] 2 (aq) + 2 e Zn (s) + 4 OH (aq) and Zn 2+ (aq) + 2e Zn(s) to calculate the value of K formation for the...
-
Thor Bhd. (Thor) is a listed company in Malaysia, specializes in selling batteries. At 31 December 2021, Thor holds four distinct types of batteries in its warehouse. The accountant of Thor provided...
-
Determine the average rate of return for a project that is estimated to yield total income of $72,000 over three years, has a cost of $125,000, and has a $25,000 residual value.
-
Pursuant to your e-mail of the 4th, please be advised that your shipment was sent March 6. Revise the following to make the tone conversational yet professional.
-
How does the addition of inert gases affect the degree of conversion of a chemical reaction?
-
Sokov Companys income statement information follows. The average number of shares outstanding was 9,600 for 2014 and 8,000 for 2013. Required Compute the following ratios for Sokov for 2014 and 2013...
-
The Scorpion King loaned money to the Mummy and his wife, Anck-su-namun. Unfortunately, the Mummy became ill and died before he repaid the loan. After three months, the Scorpion King informed...
-
In 1937, R. Schwartz and M. Schmiesser prepared a yellow-orange bromine oxide (BrO 2 ) by treating Br 2 with ozone in a fluorocarbon solvent. Many years later, J. Pascal found that, on heating, this...
-
The reaction occurring in the cell in which Al 2 O 3 and aluminum salts are electrolyzed is Al 3+ (aq) +3 e Al(s). If the electrolysis cell operates at 5.0 V and 1.0 10 5 A, what mass of aluminum...
-
Financial institutions usually require collateral as part of a lending agreement. For example, loans to build shopping centers usually require the property to be put up as collateral in case the...
-
There is a small but growing movement known as voluntary simplicity, which is founded on the belief in a simple life of working less and spending less. Do Americans who belong to this movement follow...
-
If a monopolistic competitor is able to restrict output, why doesnt it earn economic profits?
-
Say that the ultimatum game described in the chapter was changed so that the first individual could keep the money regardless of whether the offer was accepted by the second individual or not. a....
-
Distinguish the basis of judgment for the Standard Oil and the ALCOA cases.
-
Read the following article about land tenure issues in South East Asia: http://www.fao.org/docrep/009/a0306t/A0306T04.htm#ch3. What connections can you draw between the discussion in this chapter on...
-
Occupational fraud and abuse includes any personal enrichment that results from misuse or misapplication of the employing organizations resources or assets. There are four key elements to this...
-
A consumer magazine is evaluating five brands of trash compactors for their effectiveness in reducing the volume of typical household products that are discarded. In the experiment, each block...
-
This problem involves the design of a parallel adder-subtracter for 8-bit numbers expressed in sign and magnitude notation. The inputs X and Y are in sign and magnitude, and the output Z must be in...
-
Design a multiplier that will multiply two 16-bit signed binary integers to give a 32-bit product. Negative numbers should be represented in 2s complement form. Use the following method: First...
-
The objective of this problem is to use Verilog to describe and simulate a multiplier for signed binary numbers using Booths algorithm. Negative numbers should be represented by their 2s complement....
-
Financial assets with higher risk usually provide higher returns. Can this key principle get violated? Would a high risk + low return financial asset ever be priced higher than a low risk + high...
-
A stock with a beta of 1.9 has an expected rate of return of 28%. If the market return this year turns out to be 12 percentage points below expectations, what is your best guess as to the rate of...
-
Here are the returns on two stocks. Digital Cheese Executive Fruit January +14 +7 February -3 +1 March +5 +4 April +7 +13 May -4 +2 June +3 +5 July -2 -3 August -8 -2 Required: a-1. Calculate the...

Study smarter with the SolutionInn App


