Use the standard reduction potentials (Appendix M) for the half-reactions [AuCl 4 ] (aq) + 3
Question:
Use the standard reduction potentials (Appendix M) for the half-reactions [AuCl4]−(aq) + 3 e− → Au(s) + 4 Cl−(aq) and Au3+(aq) + 3 e− → Au(s) to calculate the value of Kformation for the complex ion [AuCl4]−(aq).
Data given in Appendix M
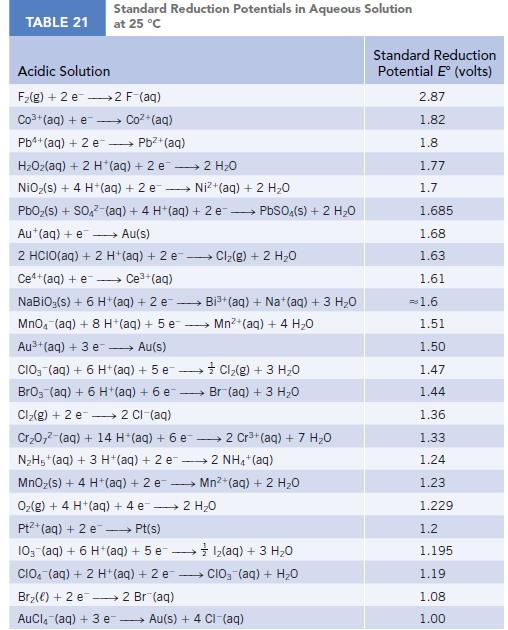
Transcribed Image Text:
TABLE 21 Standard Reduction Potentials in Aqueous Solution at 25 °C Acidic Solution F₂(g) + 2 e 2 F-(aq) Co3+ (aq) + e Coz+(aq) Pb4+ (aq) + 2 e - Pb²+(aq) HzOz(aq) + 2 H*(aq) +2e → 2H2O NiO₂(s) + 4 H+ (aq) + 2 e→→→→→→ Ni+(aq) + 2 HO PbO₂ (s) + SO4² (aq) + 4 H+ (aq) + 2e → PbSO4(s) + 2 H₂O Au+ (aq) + e→→→→→ Au(s) 2 HCIO(aq) + 2 H+ (aq) + 2 e-- - Ce+(aq) + e→→→→ Ce³+ (aq) NaBiO;(s) + 6 H+ (aq) + 2 e- → MnO4 (aq) + 8 H+ (aq) + 5 e Au³+ (aq) + 3 e→→→→→ Au(s) CIO (aq) + 6 H+ (aq) +5 e→→→→→→ BrO3 (aq) + 6 H+ (aq) + 6 e- Cl₂(g) + 2 e 2 Cl-(aq) Cr₂0,² (aq) + 14 H*(aq) + 6 e 2 Cr³+ (aq) + 7 H₂O N₂H5+ (aq) + 3 H+ (aq) + 2 e2 NH4+ (aq) MnO₂ (s) + 4 H+ (aq) + 2 e O₂(g) + 4 H+(aq) + 4 e 2 H₂O Pt+ (aq) + 2 e →→→→→ Pt(s) 10- (aq) + 6 H+ (aq) + 5 e1₂(aq) + 3 H₂O CIO (aq) + 2 H+ (aq) + 2 e CIO₂ (aq) + H₂O Br₂() +2 e 2 Br(aq) AuCl(aq) + 3 e → Cl₂(g) + 2 H₂O → Bi³+ (aq) + Na+ (aq) + 3 H₂O Mn²+ (aq) + 4H₂O Cl₂(g) + 3 H₂O Br (aq) + 3 H₂O Mn²+ (aq) + 2 H₂O Au(s) + 4 CI-(aq) Standard Reduction Potential E (volts) 2.87 1.82 1.8 1.77 1.7 1.685 1.68 1.63 1.61 = 1.6 1.51 1.50 1.47 1.44 1.36 1.33 1.24 1.23 1.229 1.2 1.195 1.19 1.08 1.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the formation constant Kf for the complex ion AuCl4aq we need to use the following equa...View the full answer

Answered By

Vineet Kumar Yadav
I am a biotech engineer and cleared jee exam 2 times and also i am a math tutor. topper comunity , chegg India, vedantu doubt expert( solving doubt for iit jee student on the online doubt solving app in live chat with student)
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Use the standard reduction potentials (Appendix M) for the half-reactions [Zn(OH) 4 ] 2 (aq) + 2 e Zn (s) + 4 OH (aq) and Zn 2+ (aq) + 2e Zn(s) to calculate the value of K formation for the...
-
Use the standard reduction potentials to find the equilibrium constant for each of the following reactions at 25°C: (a) (b) (c) Br2(1) + 21-(aq )- 2Br_(aq) + 12(s) 5Fe2 + (aq) +MnO4 (aq ) + 8H +...
-
Use the standard reduction potentials (Appendix M) for the half-reactions Hg 2 Cl 2 (s) + 2e 2 Hg() + 2 Cl (aq) and Hg 2 2+ (aq) + 2 e 2 Hg() to calculate the value of K sp for Hg 2 Cl 2 . Data...
-
Raheem & Co. purchased a fixed asset on 1.4.2018 for Rs.2,50,000. Depreciation is to be provided @10% annually according to the Straight-line method. The books are closed on 31st March every year....
-
A project has estimated annual net cash flows of $150,000. It is estimated to cost $885,000. Determine the cash payback period.
-
Repeat Problem 16-11 when \(Z_{\mathrm{L}}\) is a \(30-\Omega\) resistor in parallel with an impedance of \(30-j 30 \Omega\). Data From Problem 16-11 In Figure P16-11, the load \(Z_{\mathrm{L}}\) is...
-
The plaintiff, Thelma Agnes Smith, lived with the defendant out of wedlock for several years. When the relationship ended, she sued the defendant, seeking to enforce two written agreements with him...
-
Nelson Company, organized in 2014, has the following transactions related to intangible assets . Instructions Prepare the necessary entries to record these intangibles. All costs incurred were for...
-
Prove that if M is a dense linear subspace of a separable Hilbert space H, then H has an orthonormal basis consisting of elements in M. Does the same result hold for arbitrary dense subsets of H?
-
In 1937, R. Schwartz and M. Schmiesser prepared a yellow-orange bromine oxide (BrO 2 ) by treating Br 2 with ozone in a fluorocarbon solvent. Many years later, J. Pascal found that, on heating, this...
-
The reaction occurring in the cell in which Al 2 O 3 and aluminum salts are electrolyzed is Al 3+ (aq) +3 e Al(s). If the electrolysis cell operates at 5.0 V and 1.0 10 5 A, what mass of aluminum...
-
Examine CooperHall Companys statement of cash flows that follows. Suppose CooperHalls operating activities provided, rather than used, cash. Identify three things if using the indirect method that...
-
How do theories of legitimate authority, such as Habermas's communicative action theory and Arendt's concept of participatory democracy, offer alternative models for organizing collective...
-
What is the role of an accountant in analyzing and interpreting financial data? How does an accountant contribute to risk management and internal controls within an organization?
-
Prepare journal entries. 1. Pledges amounting to $200,000 were received. Of this amount, $50,000 was restricted for a special education research program. All of the restricted pledges and $140,000 of...
-
What are the implications of social influence and conformity on individual decision-making processes within group settings, and how do these phenomena shape collective behaviors and societal norms?
-
Function main() of a C++ program is: int main ( ) { int marks[100], numStudents, average, best, m; cout < >numStudents; Obtain( marks, numStudents); cout <
-
Besides maintaining a private law practice, Keith Gill offered credit repair services to consumers in a business that he operated with a retired attorney, Richard Murkey. In various contexts, Gill...
-
r = 0.18 Find the coefficients of determination and non-determination and explain the meaning of each.
-
This problem concerns the design of a divider for unsigned binary numbers that will divide a 16-bit dividend by an 8-bit divisor to give an 8-bit quotient. Assume that the start signal (ST = 1) is 1...
-
Design a 4 Ã 4 keypad scanner for the following keypad layout. (a) Assuming only one key can be pressed at a time, find the equations for a number decoder given R 3-0 and C 3- 0 , whose output...
-
Four pushbuttons (B 0 , B 1 , B 2 , and B 3 ) are used as inputs to a logic circuit. Whenever a button is pushed, it is debounced, after which the circuit loads the button number in binary into a...
-
Matt is the 100% owner of Zboy, Inc. Zboy paid Matt $50,000 in 2023 and also reported a taxable loss of $20,000. Assume the corporate tax rate is 21% and Matt's marginal tax rate is 22%. (also assume...
-
KRMY-TV is contemplating a T-shirt advertising promotion. Monthly sales data from T-shirt shops marketing the Eye Watch KRMY-TV design indicate that Q = 1,500 200 P where Q is T-shirt sales and P is...
-
If net sales are $300,000, cost of goods available for sale is $280,000, and gross profit percentage is 35%. 1. What is the amount of ending inventory? 2. During the months of January and February,...

Study smarter with the SolutionInn App


