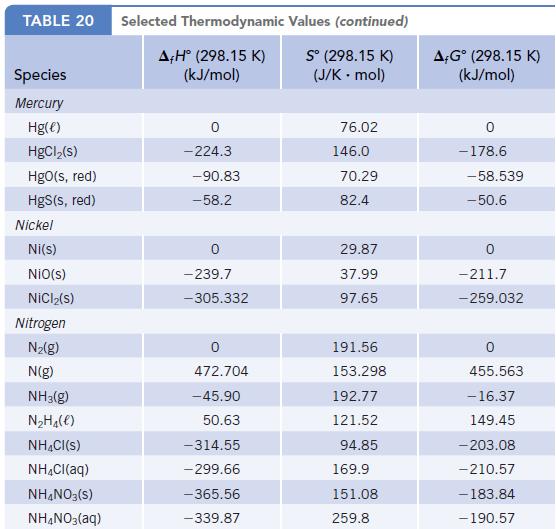
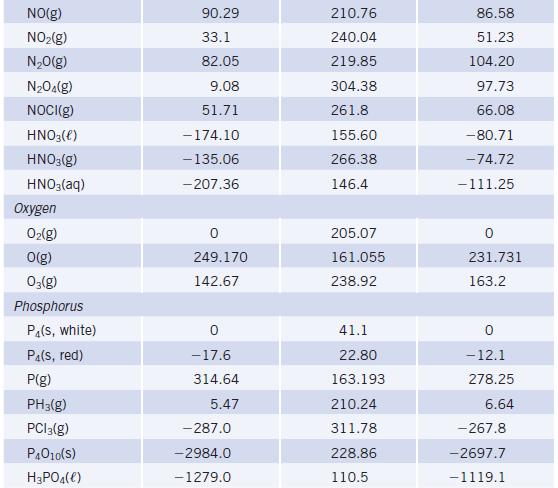
Using enthalpy of formation data in Appendix L, determine whether the decomposition of NH 4 NO 3
Question:
Using enthalpy of formation data in Appendix L, determine whether the decomposition of NH4NO3(s) to give N2O(g) and H2O(g) is endothermic or exothermic.
Data given in Appendix L
Transcribed Image Text:
TABLE 20 Species Mercury Hg(e) HgCl₂(s) HgO(s, red) HgS(s, red) Nickel Ni(s) NiO(s) NiCl₂(s) Nitrogen N₂(g) N(g) NH3(g) N₂H₁(e) NH4Cl(S) NH₂Cl(aq) NH4NO3(S) NH4NO3(aq) Selected Thermodynamic A+Hº (298.15 K) (kJ/mol) 0 -224.3 -90.83 -58.2 -239.7 -305.332 472.704 -45.90 50.63 -314.55 - 299.66 -365.56 -339.87 Values (continued) S° (298.15 K) (J/K - mol) 76.02 146.0 70.29 82.4 29.87 37.99 97.65 191.56 153.298 192.77 121.52 94.85 169.9 151.08 259.8 A-Gº (298.15 K) (kJ/mol) 0 -178.6 -58.539 -50.6 -211.7 -259.032 455.563 -16.37 149.45 -203.08 -210.57 -183.84 - 190.57
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
NHNO 30 NO 2HO AH for NH NO 36556 KJmol 300 AH f...View the full answer

Answered By

Chiranjib Thakur
I have no tutoring experience yet, but I can share my skills and knowledge gained from my education and work experiences. I have been a CPA since 2012 with 6 years of work experience in internal auditing and 4 years of work experience in accounting at the supervisory level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Accordingly, although ISAs do not impose responsibilities on management and those charged with governance and do not override laws and regulations that govern their responsibilities, ISAs are...
-
Use data in Appendix L to calculate the enthalpy and free energy change for the reaction 2 NO 2 (g) N 2 O 4 (g) Is this reaction exothermic or endothermic? Is the reaction product- or...
-
Use data in Appendix L to calculate the enthalpy and free energy change for the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g). Is this reaction exothermic or endothermic? Is the reaction product- or...
-
On December 31, 2021, Tiny Tims Tech, Inc. a private company who follows ASPE, leased a colour copier from Cory Copy Corporation at a price of $479,079. The lease agreement specifies annual payments...
-
Colleges and universities have a variety of internal and external customers. Use a team of three or four individuals to answer the following: a. Who are three internal and two external customers of a...
-
In 1970, __________ _____ issued the first MBSs in the United States when it sold securities backed by its FHA and VA loans.
-
\(C_{P}-C_{V}=R\) is valid for (a) Real gases (b) All gases (c) Ideal gases (d) None of these.
-
1. Which leadership style would Fiedler say Li Chang uses? 2. Using Exhibit 4.3, Fiedlers contingency leadership model, what situation and leadership style are appropriate for the production...
-
Based on the following information, what is C/G/S for Moroni Industries. Inc. using FIFO under the periodic inventory method? Beginning Inventory 10 @ $120; Purchase 60 @ $112, Sale 40; Purchase 30 @...
-
The chemistry of gallium: (a) Gallium hydroxide, like aluminum hydroxide, is amphoteric. Write a balanced equation to show how this hydroxide can dissolve in both HCl(aq) and NaOH(aq). (b) Gallium...
-
When palladium metal is exposed to H 2 gas, the metal become brittle because H 2 molecules dissociate and H atoms fill some of the octahedral holes in the face-centered cubic lattice. To find the...
-
State two properties that distinguish a superconductor from a normal metallic conductor.
-
Some people are about to form a company, as a vehicle through which to run a new business. What factors should they bear in mind when deciding between forming a private limited company or a public...
-
Compare the main features of a preference share with those of: (a) an ordinary share; and (b) loan notes.
-
Should favourable variances be investigated to discover their cause?
-
The evidence shows that more than half of the shares listed on the London Stock Exchange are owned by investors who are based overseas. Why might overseas investors want to buy shares listed in...
-
Surveys show that many small businesses use credit cards to help finance their activities. Why might they do this?
-
What are the differences between a forged maker and a forged endorsement scheme?
-
A police officer pulls you over and asks to search your vehicle because he suspects you have illegal drugs inside your car. Since he doesn't have reasonable suspicion to search your car, legally he...
-
A candle flame is 18.0 cm in front of a thin positive lens. Its image appears three times farther away from the lens than if the same candle were on a very distant mountain. Determine the lenss focal...
-
What must the focal length of a thin negative lens be for it to form a virtual image 50 cm away (measured from the lens) of an ant located 100 cm away (measured from the lens)? Given (just as a...
-
An LED is on the central axis 30.0 cm in front of a thin lens. The resulting image, which is virtual, is 10.0 cm from the lens. Determine the focal length of the lens. Using Table 5.3, explain why...
-
B. Complete the Chart sections A, B, and C using the part you created. 1. What is the diameter of the allowed deviation from true position (column A)? 2. What is the diameter of the deviation zone...
-
A structure consists of three initially-undeformed springs, as shown in the figure below. Use the following values: k = k = k3 = 5000 lbf/in, P2 1000 lbf, and P4 = 4000 lbf. Solve by hand. 1. Compute...
-
Q-4 (30 pts.) Superheated steam enters a turbine at 2 MPa and 600C and exits at 10 kPa. For a steam mass flow rate of 2 kg/s, determine (a) (15p) turbine inlet and isentropic exit conditions...

Study smarter with the SolutionInn App


