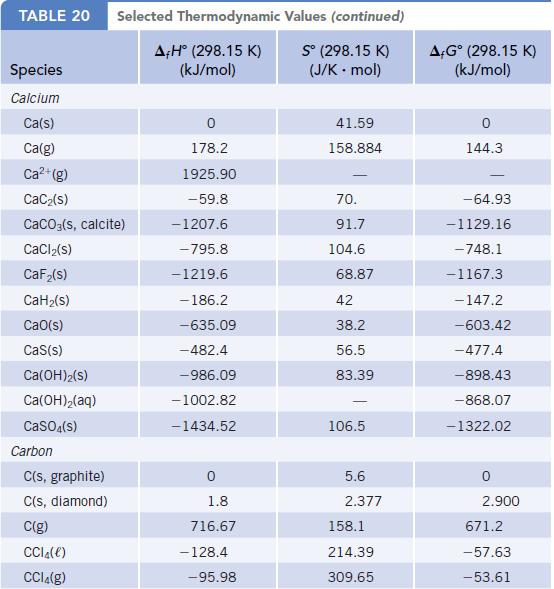
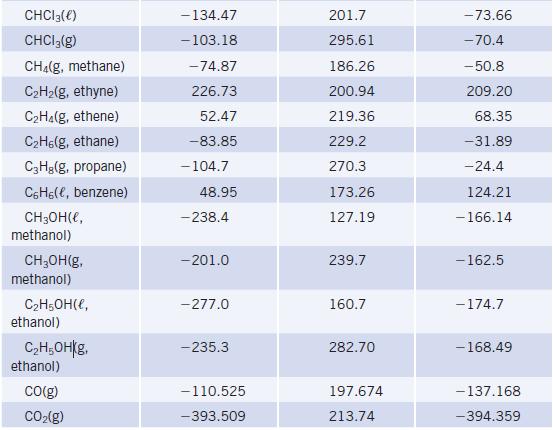
Using thermodynamic data, estimate the normal boiling point of ethanol. (Recall that liquid and vapor are in
Question:
Using thermodynamic data, estimate the normal boiling point of ethanol. (Recall that liquid and vapor are in equilibrium at 1.0 atm pressure at the normal boiling point.) The actual normal boiling point is 78°C. How well does your calculated result agree with the actual value?
Thermodynamic Data
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





