A constant current of 0.500 A passes through a silver nitrate solution for 90.0 minutes . What
Question:
A constant current of 0.500 A passes through a silver nitrate solution for 90.0 minutes .
What mass of silver metal is deposited at the anode?
Strategy First, determine the half-reaction involved. Then, determine the total charge of the process and relate that to the stoichiometry of the half-reaction to determine the mass of silver deposited. The flow diagram below shows the specific steps.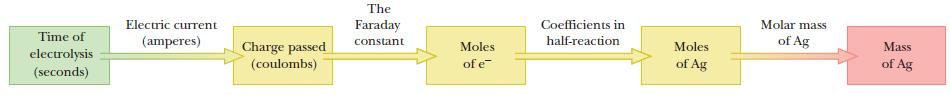
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





