Question: Consider the formation of double-stranded (DS) DNA from two complementary single strands (S and S²) through the following mechanism involving an intermediate helix (IH): a.
Consider the formation of double-stranded (DS) DNA from two complementary single strands (S and S€²) through the following mechanism involving an intermediate helix (IH):
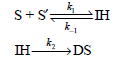
a. Derive the rate law expression for this reaction employing the preequilibrium approximation.
b. What is the corresponding rate law expression for the reaction employing the steady-state approximation for the intermediate IH?
S+S=H DS -
Step by Step Solution
3.40 Rating (162 Votes )
There are 3 Steps involved in it
a The rate law expression for the reaction is We apply the steadystate approximation to defin... View full answer

Get step-by-step solutions from verified subject matter experts


