Draw an energy-level diagram that represents the Hesss law calculation in Exercise 5.71. Exercise 5.71 Given the
Question:
Draw an energy-level diagram that represents the Hess’s law calculation in Exercise 5.71.
Exercise 5.71
Given the thermochemical equations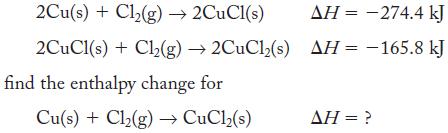
Transcribed Image Text:
2Cu(s) + Cl₂(g) → 2CuCl(s) 2CuCl(s) + Cl₂(g) →2CuCl2(s) find the enthalpy change for Cu(s) + Cl₂(g) → CuCl₂(s) AH = -274.4 kJ AH = -165.8 kJ AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Cus Clg 137...View the full answer

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Draw an energy level diagram for the chemical reaction in Exercise 9. In exercise 9, Nitrogen gas reacts with oxygen gas to make NO(g) while absorbing 180 kJ. Is this process exothermic or...
-
The enthalpy changes of the following reactions can be measured: (a) Use these values and Hesss law to determine the enthalpy change for the reaction (b) Draw an energy level diagram that shows the...
-
The enthalpy changes for the following reactions can be measured: (a) Use these values and Hesss law to determine the enthalpy change for the reaction (b) Draw an energy level diagram that shows the...
-
What other advice can you offer owners of seasonal businesses about coping with the effects of their companies highly irregular sales patterns? About managing cash flow in general?
-
Estimate the sphericities of the following simple particle shapes: (a) a cylindrical needle with a height, H, equal to 5 times the diameter, D (b) a rectangular prism of sides a, 2a, and 3a
-
Verify the formula differentiation. [(si (sin x) dx = x(sin x) - 2x + 2V1x2 sinx + C
-
Refer to the data presented in exercise 8.2. Assume further that Price and Stewart agree that their opening capital balances in the new partnership should be the same and set the amount at $380 000....
-
Emerald Island Company is considering building a manufacturing plant in County Kerry. Predicting sales of 100,000 units, Emerald Isle estimates the following expenses: An Irish firm that specializes...
-
31 The number of protons, electrons and neutrons in aluminium ion Al+ is Protons A. 27 B. 13 C. ABCD 32 32. D. 13 10 Electron 27 neutrons 14 14 14 10 14 17 14 The formula of the compound formed...
-
1. Supply the missing income statement amounts for each of the following companies for the year ended December 31, 2017: 2. Prepare the income statement for Bell Co., which uses the periodic...
-
Draw an energy-level diagram that represents the Hesss law calculation in Exercise 5.72. Exercise 5.72 In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as...
-
Given the thermochemical equations 2Cu(s) + Cl(g) 2CuCl(s) 2CuCl(s) + Cl(g) 2CuCl2(s) find the enthalpy change for Cu(s) + Cl(g) CuCl(s) AH = -274.4 kJ AH = -165.8 kJ AH = ?
-
What advice do you have for colleagues regarding workfamily balance?
-
A chemical reaction is found to be 15 times faster at \(100^{\circ} \mathrm{C}\) than at \(25^{\circ} \mathrm{C}\). Measurements show that the pre-exponential term contains temperature to the power...
-
(a) What is meant by the terms (i) a global reaction; (ii) an elementary reaction; (iii) a reaction mechanism. (b) Describe the steps required to form a chain reaction and explain why chain reactions...
-
An engine working on the constant volume (Otto) cycle has a compression ratio of 6.5 to 1 , and the compression follows the law \(p V^{1.3}=\mathrm{C}\), the initial pressure and temperature being 1...
-
A \(10 \%\) rich mixture of heptane \(\left(\mathrm{C}_{7} \mathrm{H}_{16} ight)\) and air is trapped in the cylinder of an engine at a pressure of \(1 \mathrm{bar}\) and temperature of \(400...
-
A turbocharged, intercooled compression ignition engine is operated on octane \(\left(\mathrm{C}_{8} \mathrm{H}_{18} ight)\) and achieves constant pressure combustion. The volumetric compression...
-
Respond briefly to the following statement: You say stock price equals the present value of future dividends? Thats crazy! All the investors I know are looking for capital gains.
-
Data on weekday exercise time for 20 females, consistent with summary quantities given in the paper An Ecological Momentary Assessment of the Physical Activity and Sedentary Behaviour Patterns of...
-
Why do deviations from ideal behavior occur at lower concentrations for electrolyte solutions than for solutions in which the solute species are uncharged?
-
Why is the value for the dielectric constant for water in the solvation shell around ions less than that for bulk water?
-
What can you conclude about the interaction between ions in an electrolyte solution if the mean ionic activity coefficient is greater than one?
-
Why should investors who identify positive-NPV trades be skeptical about their findings if they don't inside information or a competitive advantage? What return should the average investor expect to...
-
What is the beta of a stock that begins with the same letter as your first name? What is the beta of a stock that begins with the same letter as your last name? Why is it so common to use historical...
-
Many families rent their living quarters. So how much does it cost to rent an apartment? Find TWO apartments in the same zip code. Compare and contrast each unit. Supply the following information:...

Study smarter with the SolutionInn App


