For each of the reactions, calculate E from the table of standard potentials, and state whether the
Question:
For each of the reactions, calculate E° from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard conditions.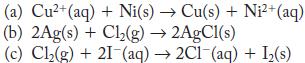
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate E standard potential for each reaction and determine if the reaction is spontaneous as ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
For each of the reactions, calculate E from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard...
-
Calculate the potential for each of the voltaic cells in Exercise 18.44 when the concentrations of the soluble species and gas pressures are as follows: Exercise 18.44 For each of the reactions,...
-
(a) LEP Table 12-2: Exothermic Reaction with Heat Exchange Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase...
-
Blaine is a practicing dentist. He operates his business from the basement of his house, with a separate entrance and facilities. Blaine uses the simplified method every year to calculate his...
-
The flat roof on the refrigeration compartment of a food deli very truck is of length L = 5 m and width W = 2 m. It is fabricated from thin sheet metal to which a fiberboard insulating material of...
-
In the text it was stated that when a mobile host is not at home, packets sent to its home LAN are intercepted by its home agent on that LAN. For an IP network on an 802.3 LAN, how does the home...
-
Large rotating machinery has historically been fitted with sleeve bearings. Manufacturers have switched to ball bearings to lower cost. The clearances in these bearings can be so large that the balls...
-
Mandy Arnold opened a law office, Mandy Arnold, Attorney at Law, on July 1, 2014. On July 31, the balance sheet showed Cash $4,000, Accounts Receivable $1,500, Supplies $500, Equipment $5,000,...
-
A dipole with dipole moment p = 2 + 4 j is placed in an electric field E = 7 i - 1 0 j , with p in Cm and E in N / C . The dipole is allowed to move freely. What will the potential energy of the...
-
Use the data from the table of standard reduction potentials in Appendix H to calculate the standard potential of the cell based on each of the following reactions. In each case, state whether the...
-
Two electrodes are immersed in a 1 M HBr solution. One of the electrodes is a silver wire coated with a deposit of AgBr(s). Th e second electrode is a platinum wire in electrical contact with a...
-
For the following exercises, determine if the given graph is a one-to-one function. aquaquagado HI
-
Tesco.com has established a position of being the worlds leading online grocer with an estimated sales turnover of 401 million and profits up 37 per cent to 21 million (as at 21 September 2005)....
-
Describe and briefly explain the purpose of the different stages involved in updating an existing document on a commercial website.
-
Describe different options for testing the effectiveness of competing offers online.
-
A service is rented rather than owned. Explain what this statement means, and use examples to support your explanation.
-
From a resource-based view, explain the difference between resources and capabilities.
-
(Multiple Choice) Questions 13 are based on the following facts: Fred Harris began a music business in July 2012. Harris prepares monthly financial statements and uses the accrual basis of...
-
What key concerns must functional tactics address in marketing? Finance? POM? Personnel?
-
Pulling gs. Suppose again you are the astronaut in Problem 9. When most people are subjected to an acceleration greater than about 5 g, they will usually become unconscious (black out). Will you be...
-
Consider a string with one end tied to a tall ceiling and the other end hanging freely. Explain why the tension at the bottom of the string is smaller than the tension at the top.
-
Draw a qualitative plot of the total force acting on the ball in Figure 3.15 (page 68) as a function of time. Begin your plot while the ball is still in the throwers hand and end it after the ball...
-
How do advanced organizations cultivate a conducive ecosystem that fosters creativity and innovation, leveraging sophisticated methodologies to curate dynamic work environments that stimulate...
-
1. What is the essential basis for ethical reasoning, according to Paul and Elder? Why are ethical principles not a matter of subjective preference? 2. How might reasoning processes be used to...
-
As preparation for the final research paper, formulate a theory about the correlation between a measurable dependent variable (the effect) and measurable independent variables (the causes). The topic...

Study smarter with the SolutionInn App


