Given the thermochemical equations Sn(s) + Cl(g) SnCl(s) SnCl,(s) + Cl,(g) SnCl(0) determine AH for
Question:
Given the thermochemical equations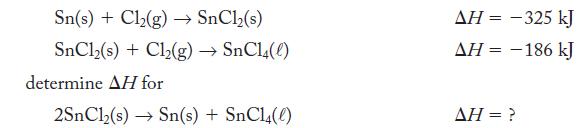
Transcribed Image Text:
Sn(s) + Cl₂(g) → SnCl₂(s) SnCl,(s) + Cl,(g) → SnCl(0) determine AH for 2SnCl₂(s)→ Sn(s) + SnC14(0) AH = -325 kJ ΔΗ = − 186 kJ - AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Hamza Amjad
Currently I am student in master degree program.from last two year I am tutring in Academy and I tought many O/A level student in home tution.
4.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
From the following data, calculate : (a) P/V Ratio. (b) Profit when sales are Rs. 40,000. (c) New break-even point if selling price is reduced by 20%. Fixed Expenses Rs. 8,000. Break-Even point Rs....
-
One way to evaluate fuels with respect to global warming is to determine how much heat they release during combustion relative to how much CO 2 they produce. The greater the heat relative to the...
-
Using the thermochemical equations in Exercise 5.67 as needed and in addition Exercise 5.67 Using the following thermochemical equations CH4(g) + 20(g) CO(g) + 2HO(l) CH4(g) + 30(g) 2CO(g) + 2HO(l)...
-
Three employees of the Horizon Distributing Company will receive annual pension payments from the company when they retire. The employees will receive their annual payments for as long as they live....
-
In Example 15.6, pure-component, liquid-phase adsorption data are used with the extended-Langmuir isotherm to predict a binary-solute data point. Use the following mixture data to obtain the best fit...
-
Baseball player Robinson Cano is asking for salary arbitration on his contract. Salary arbitration in Major League Baseball works as follows: The player submits a salary that he thinks he should be...
-
Suppose that you purchase the forklift for $20,000 by borrowing the purchase price using a four-year annuity loan. What would the monthly loan payment be in a perfect capital market where the...
-
Fox Enterprises is considering expanding into the growing laser copier business. Fox estimates that this expansion will cost $1.8 million and will generate a 20-year stream of expected net cash flows...
-
Your 3 year old nephew got into the workbench and mixed iron filings into a container with sugar and marbles. Explain the steps you would take to separate and recover each substance (use point form...
-
You have been hired as the new controller for Paulson Paint, Inc. You will have the opportunity to utilize your financial and managerial accounting experience. You will be responsible for preparing...
-
Describe the difference between the system and the surroundings.
-
Th e chemical industry converts hydrocarbons of low molecular mass to larger and more useful compounds. Calculate the change in enthalpy for the synthesis of cyclohexane (C 6 H 12 ), a compound used...
-
Distinguish between the stand-alone and the incremental revenue-allocation methods.
-
For the Ewens distribution (12.5), show that the conditional distribution given \(\# B=k\) is \[ p_{\theta}(B \mid \# B=k)=\frac{\prod_{b \in B} \Gamma(\# b)}{s_{n, k}} \] where \(s_{n, k}\) is...
-
A thermocouple is connected across a battery, and a current flows through it. The cold junction is connected to a reservoir at \(0{ }^{\circ} \mathrm{C}\). When its hot junction is connected to a...
-
The emf of a copper-iron thermocouple with its cold junction at \(0{ }^{\circ} \mathrm{C}\) is given by \[\varepsilon=-13.403 t+0.0138 t^{2}+0.0001 t^{3} \quad \mu \mathrm{V}\] where \(t=\)...
-
A fluid consisting of a single component is contained in two containers at different temperatures. Show that the difference in pressure between the two containers is given by \[\frac{\mathrm{d}...
-
Carrie Raymond is a first-year associate at a large criminal defense law firm in Philadelphia. The firm recently received a new clienta famous football player charged with extortion. The partner on...
-
Why is quantitative valuation of real options often difficult in practice? List the reasons briefly.
-
Define a traverse in Surveying?
-
Draw a complete mechanism for the following transformation. NaOH, heat
-
Identify the reagents you would use to prepare the following compound via a Robinson annulation. . 0=
-
Using cyclopentanone as your starting material and using any other reagents of your choice, propose an efficient synthesis for each of the following compounds. (a) (b) (c) OH HO.
-
Continuing with the prior problem assume that the year has ended, and Myles Corporation experienced the following revenues and total costs: Total revenue $2,700,000 Total variable material costs...
-
The 2013 financial statements for BNSF Railway report the following information: Year ended December 31, 2013 2012 (In millions) Revenues $22,014 $20,835 Property and equipment, net 52,363 50,070...
-
What is the firm's cash flow from financing, using the data below? Net Income $1000 Depreciation Expense $300 Change in operating assets $600 Change in net PP&E $5000 Change in long-term Liabilities...

Study smarter with the SolutionInn App


