Th e chemical industry converts hydrocarbons of low molecular mass to larger and more useful compounds. Calculate
Question:
Th e chemical industry converts hydrocarbons of low molecular mass to larger and more useful compounds. Calculate the change in enthalpy for the synthesis of cyclohexane (C6H12), a compound used in the production of nylon, from ethylene.![]()
Use the information in Example 5.8 and the thermochemical equation for the combustion of cyclohexane:![]()
Strategy
Arrange the thermochemical equations so that the reactant, C2H4, is on the left and the product, C6H12, is on the right. The other products and reactants should all cancel so that the desired reaction is all that remains.
Example 5.8
Hydrogenation of hydrocarbons is an important reaction in the chemical industry. A simple example is the hydrogenation of ethylene to form ethane. Calculate the enthalpy change for ![]()
Use the following thermochemical equations to determine the overall enthalpy change.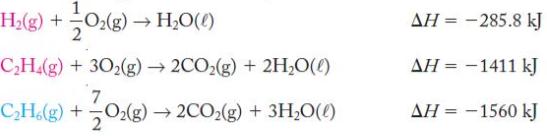
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





