Hydrazine, N 2 H 4 , is used as a fuel in some rockets: What is the
Question:
Hydrazine, N2H4, is used as a fuel in some rockets: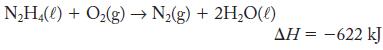
What is the enthalpy change if 110.0 g N2H4 reacts with excess oxygen?
Transcribed Image Text:
N₂H4(l) + O₂(g) → N₂(g) + 2H₂O(l) ΔΗ = −622 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The given reaction is N2H41 O2g N2g 2H2O1 AH 622 kJ First we need to fin...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Hydrazine (N 2 H 4 ) is used as a fuel in liquid-fueled rockets. When hydrazine reacts with oxygen gas, nitrogen gas and water vapor are produced. Write a balanced equation and use bond energies from...
-
The steering rockets in space vehicles use N 2 O 4 and a derivative of hydrazine, 1,1-dimethylhydrazine (Study Question 5.86). This mixture is called a hypergolic fuel because it ignites when the...
-
What is a conversion rate and why is it so important to marketers? Provide an example of how an organization can improve this rate.
-
What is the smallest (most negative) 32-bit binary number that can be represented with (a) Unsigned numbers? (b) Twos complement numbers? (c) Sign/magnitude numbers?
-
Fifty-thousand pounds per hour of a 20 wt% aqueous solution of NaOH at 120F is to be fed to an evaporator operating at 3.7 psia, where the solution is concentrated to 40 wt% NaOH. The heating medium...
-
Describe Newtons method for solving equations. Give an example. What is the theory behind the method? What are some of the things to watch out for when you use the method?
-
Suppose a company can replace the packing material it currently uses with a biodegradable packing material. The company believes this move to biodegradable packing materials will be well received by...
-
Olstad Company issued $350,000 of 8%, 20-year bonds on January 1, 2012, at face value. Interest is payable annually on January 1. Instructions Prepare the journal entries to record the following...
-
Step 2: Smooth Sailing 1. Edit the sshd_config file. [Your bash commands here]
-
Effective January 1, 1970, Chrysler Corporation adopted the FIFO method for inventories previously valued by the LIFO method. The 1970 annual report stated, This . makes the financial statements with...
-
The combustion of 1.00 mol liquid octane (C 8 H 18 ), a component of gasoline, in excess oxygen is exothermic, producing 5.46 10 3 kJ of heat. (a) Write the thermochemical equation for this...
-
The thermite reaction produces a large quantity of heat, enough to melt the iron metal that is a product of the reaction: What is the enthalpy change if 50.0 g Al reacts with excess iron(III) oxide?...
-
State the name of the property illustrated. (3 a) b = 3 (a b)
-
Jill Parks was a healthy, vibrant 22-year-old college student embarking on her senior year. One evening after drinking at a local bar with her friends, Jill was crossing the street when an oncoming...
-
Michael Janson is a local police officer. One evening, Janson pulled over George McCain for making an illegal lane change without a signal. Janson insisted that George exit the vehicle and searched...
-
Mark is a self-appointed computer science expert. He decides one day to challenge himself and see if he can hack into the U.S. Department of Defense website just for kicks. Mark has no intention of...
-
One afternoon, Dan notices that his neighbors front door is wide open and no one appears to be home. Dan enters the front door to make sure that everything is OK inside. After he enters the foyer,...
-
The ignition system in a car uses a transformer whose primary has 200 turns and carries 10 A; its secondary, with 20,000 turns, is connected to the spark plug. An electronic switch suddenly stops the...
-
The annually compounded discount rate is 5.5%. You are asked to calculate the present value of a 12-year annuity with payments of $50,000 per year. Calculate PV for each of the following cases. a....
-
What is taxable income, and what is the formula for determining taxable income?
-
A more accurate expression for E osc would be obtained by including additional terms in the TaylorMacLaurin series. The TaylorMacLaurin series expansion of f (x) in the vicinity of x 0 is given by...
-
The observed lines in the emission spectrum of atomic hydrogen are given by In the notation favored by spectroscopists, = 1/ = E/hc and R H =109,677 cm 1 . The Lyman, Balmer, and Paschen series...
-
Calculate the speed that a gas-phase fluorine molecule would have if it had the same energy as an infrared photon ( = 1.00 10 4 nm), a visible photon ( = 500. nm), an ultraviolet photon ( = 100....
-
Meredith, a HIM Director, just returned from an invigorating full week meeting with several peer HIM leaders from her region. The presentations were extremely beneficial and Meredith had the...
-
You have decided to purchase 25 of 2/15/2019 put contracts on the DJI A with an exercise price of $246. Each contract is for 100 shares. "DJX (1/100 DOW JONES INDUSTRIAL AVERAGE) Jan 21, 2019 @ 15:52...
-
Pizza Hut is a restaurant franchise with headquarters in Texas, United States. The firm is renowned for its delicious, economical, accessible, and extensive selection of pizzas. Pizza Hut has...

Study smarter with the SolutionInn App


