In Example 3.15, the empirical formula of a compound extracted from tobacco was calculated to be C
Question:
In Example 3.15, the empirical formula of a compound extracted from tobacco was calculated to be C5H7N. In a separate experiment, the molar mass of this compound is found to be 162 g/mol. Calculate the molecular formula.
Strategy
The molecular formula is determined from the empirical formula by dividing the molar mass found in the experiment by the molar mass of the empirical formula and multiplying the subscripts of the empirical formula by the resultant whole number.
Example 3.15
Calculate the empirical formula of a compound extracted from tobacco. Chemical analysis shows that this substance contains 74.0% carbon , 8.70% hydrogen , and 17.3% nitrogen.
Example 3.14
Analysis of a 0.330-g sample of a compound shows that it contains 0.226 g chromium and 0.104 g oxygen. What is the empirical formula?
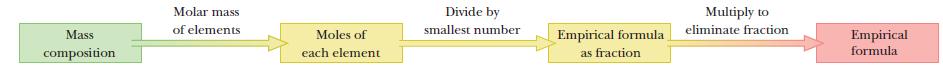
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





