Natural gas is mostly methane, CH 4 . Homes that are heated by natural gas obtain the
Question:
Natural gas is mostly methane, CH4. Homes that are heated by natural gas obtain the energy from the combustion reaction![]()
(a) Balance the reaction.
(b) Is this a redox reaction? Defend your answer.
(c) Determine the enthalpy change of this reaction using the enthalpy of formation data in the Appendix G.
(d) Determine the enthalpy change of this reaction using the bond energy data in Table 9.4. Compare your answer here with your answer in (c) and comment. Which answer do you expect is more accurate?
Table 9.4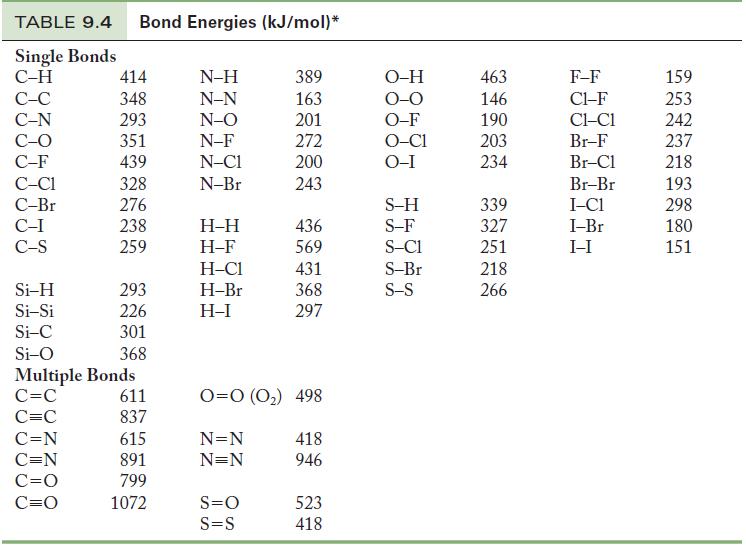
(e) Which of the bonds in the reactants and products are polar? Which are nonpolar?
(f) Most utility companies sell natural gas in units of 100 cubic feet (abbreviated ccf ), where 1 ccf = 2831 L. If the natural gas is at standard temperature and pressure, how much energy is given off by the combustion of 1 ccf natural gas?
(g) If natural gas burns in limited oxygen, carbon monoxide is the product instead of carbon dioxide:![]()
Balance this reaction.
(h) What are the diff erences in the CO bonds in CO and CO2? Assign formal charges to the atoms in each.
(i) Determine the energy change of the CO-containing reaction using data in Appendix G and the data in Table 9.4. Are your answers closer to each other than your answers in (c) and (d)?
(j) Exactly 1 mol CH4 was burned and 600.0 kJ was given off . How many grams of CO and CO2 were produced?
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





