The equilibrium constant for the following reaction is 1.0 10 14 at 298 K . Strategy
Question:
The equilibrium constant for the following reaction is 1.0 × 1014 at 298 K .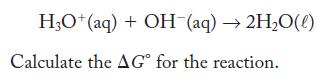
Strategy
We know that ΔG ° = -RT ln Keq, and we have values for Keq and T. Use the proper value and units for R so the units of ΔG ° are joules or kilojoules.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Using R 8314 JK and T 298 K we subst...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
You have been assigned the task of measuring the equilibrium constant for the reaction N 2 O 4 2NO 2 as a function of temperature. To do so, you evacuate a rigid 2-liter vessel equipped with a...
-
List two similarities and two differences between the Safety Analysis of the Incident Algorithm and the BowTie Diagram. a. Example 6-1: Gas-Phase Reaction in a Microreactor Wolfram and Python 1. Use...
-
The equilibrium constant for dissociation of N2O4 is 0.664 and 0.141 at 318 K and 298 K respectively. Calculate the average heat of reaction within this temperature range.
-
How does science affect the selection process? Explain your reasoning
-
Butacetin is an analgesic (pain, killing) agent that is synthesized commercially from p-fluoronitrobenzene. Propose asynthesis. NHCOCH3 Butacetin (CH3)3CO
-
The following state transition table is a simplified model of process management, with the labels representing transitions between states of READY, RUN, BLOCKED, and NONRESIDENT. Give an example of...
-
In a binomial tree with \(n\) steps, let \(f_{j}=f_{u \ldots u d \ldots d}(j\) times \(u\) and \(n-j\) times \(d\) ). For a European Call option expiring at \(T=n \Delta t\) with strike \(K\), show...
-
A hair dryer may be idealized as a circular duct through which a small fan draws ambient air and within which the air is heated as it flows over a coiled electric resistance wire. Surroundings, Tur...
-
In the previous task, we use the certificates in the /etc/ssl/certs folder to verify server's certificates. In this task, we will create our own certificate folder, and place the corresponding...
-
The standard Gibbs free-energy change for the following reaction is +55.69 kJ . Strategy Because we know the G and the temperature, we can use Equation 17.11 to determine the equilibrium constant....
-
Calculate the Gibbs free-energy change for the reaction of nitrogen monoxide and bromine to form nitrosyl bromide at 298 K under two sets of conditions. (a) The partial pressure of each gas is 1.0...
-
Suppose that the U.S. demand for maple syrup, in thousands of gallons per year, is Qd = 6000 - 30P, where P is the price per gallon. What is the elasticity of demand at a price of $75 per gallon?
-
What should financial records provide to the clinic? In your opinion.
-
How do mutualistic interactions contribute to the resilience of agricultural systems, and what strategies can be employed to enhance the sustainability and productivity of agroecosystems through the...
-
Recorded costs for Division A, which manufactured 3,000 units of Product X during the month, are as follows: Direct materials $250,000, Direct labor 200,000, Indirect production costs 40,000,...
-
Ingrid Dodson is a staffing specialist at a K-12 tutoring company. The tutoring company is expanding beyond their current writing and math tutoring services and will also offer financial tutoring...
-
An organization should follow a promote-from-within policy. What are the advantages and disadvantages of a promote-from-within policy, and when should an organization utilize this practice? Explain.
-
Multiple Choices Questions 1. Which of the following would be considered a significant deficiency in an organization's control environment? a. The internal audit function is outsourced to a public...
-
What is the order p of a B + -tree? Describe the structure of both internal and leaf nodes of a B + -tree.
-
If the accuracy of positioning the probe described in Problem 9.5 is plus or minus 5.0 mm, compute the possible error in measuring the average velocity.
-
A small velocity probe is to be inserted through a pipe wall. If we measure from the outside of the DN 150 Schedule 80 pipe, how far (in mm) should the probe be inserted to sense the average velocity...
-
Compute points on the velocity profile from the tube wall to the centerline of a standard hydraulic steel tube, 50 mm OD 1.5 mm wall, if the volume flow rate of SAE 30 oil (sg = 0.89) at 110C is 25...
-
Math 210 Weekly Proof #8 Chapter 11 - Counting Remember that when writing proofs, you will be graded both on the correctness of your logic and on the clarity of your writing. Use complete sentences,...
-
What role do cultural factors, including norms around hierarchy, communication styles, and conflict management traditions, play in shaping conflict resolution approaches and influencing the...
-
Case 17-1 Randall Corporation Paul Syrie, senior partner at Newcombe Consulting, was preparing his presentation for the CEO of a large client, Randall Corporation, the following day. Newcombe had...

Study smarter with the SolutionInn App


