The standard Gibbs free-energy change for the following reaction is +55.69 kJ . Strategy Because we know
Question:
The standard Gibbs free-energy change for the following reaction is +55.69 kJ .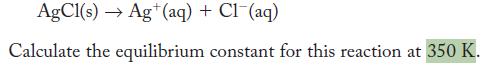
Strategy
Because we know the ΔG ° and the temperature, we can use Equation 17.11 to determine the equilibrium constant.
Equation 17.11![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
We should convert the AG to units of J AG ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Reactions between gases in the atmosphere are not at equilibrium, but for a thorough understanding of them we need to study both the rates at which they take place and their behavior under...
-
Calculate the standard Gibbs free energy change of reaction in each of the following using the standard molar values for Gibbs free energy change given here. In each case, comment on whether the...
-
Hydrogen iodide, HI, is used as a reagent in organic chemistry to transform primary alcohols into alkyl iodides. Suppose you are a chemist using HI; you would need to understand the equilibrium...
-
Recruitment and selection involves the following except: a) building a pool of candidates. b) applicants completing application forms. c) downsizing the organization. d) employment planning and...
-
Phenyl 4-aminosalicylate is a drug used in the treatment of tuberculosis. Propose a synthesis of this compound starting from 4-nitrosalicylicacid. CO2H H2N O2N Phenyl 4-aminosalicylate...
-
The Fastest (and Most Expensive) Car! The table shows test data for the Bugatti Veyron, the fastest car made. The car is moving in a straight line (the x-axis). (a) Make a v.-t graph of this car's...
-
The distribution of the ages of the winners of the Tour de France from 1903 to 2016 is approximately bell-shaped. The mean age is 27.9 years, with a standard deviation of 3.3 years. Use the...
-
Croftmark Co. began operations on May 1, 2010. Its Work in Process Inventory account on May 31 appeared as follows: The company applies overhead on the basis of direct labor cost. Only one job was...
-
A 2 . 5 kg physics book, initially at rest, is pushed 1 . 2 m along a horizontal tabletop by a horizontal force of 2 . 0 N . Assume there is no friction .Determine the average power developed by the...
-
The vapor pressure of water at 25 C is 23.77 torr , increasing to 42.20 torr at 35 C . Calculate the standard free energy and standard enthalpy changes at 25 C for the vaporization of water. Strategy...
-
The equilibrium constant for the following reaction is 1.0 10 14 at 298 K . Strategy We know that G = -RT ln K eq , and we have values for K eq and T. Use the proper value and units for R so the...
-
Discuss how the availability of disease management, training programs, and a nursing hotline might help with health benefits costs.
-
Draw EmployeeGraph, implemented as an adjacency matrix. Store the vertex values in alphabetical order. EmployeeGraph V(EmployeeGraph) E(EmployeeGraph) = (V, E) {Susan, Darlene, Mike, Fred, John,...
-
These two approaches represent the classic trade-off between space and algorithm complexity. Please comment. Exercises 29 DateType keeps only the integer representation of the month, day, and year....
-
Name three perspectives from which we can view data. Using the logical data structure a list of student academic records, give examples of what each perspective might tell us about the data.
-
Why is exhaustive data-coverage testing virtually impossible?
-
True or False? A graph vertex cannot have an edge that connects to itself.
-
How does the firm's management compensation plan change over the life cycle of the firm's products?
-
What are the typical record-at-a-time operations for accessing a file? Which of these depend on the current file record?
-
Convert 0.008 ft 3 /s to gal/min.
-
An existing fixture inserts the velocity probe described in Problem 9.5 exactly 60.0 mm from the outside surface of the pipe. If the probe reads 2.48 m/s, compute the actual average velocity of flow,...
-
An alternative scheme for using the velocity probe described in Problem 9.5 is to place it in the middle of the pipe, where the velocity is expected to be 2.0 times the average velocity. Compute the...
-
Your clients Sabine and Pavel live in a downtown condominium building near the lake. They pay monthly mortgage payments of $2,000 plus a condo fee of $450. They also pay $300 per month in property...
-
Oftentimes, managers perceive it as an attractive strategic decision to pursue the acquisition of another company. However, evidence suggests that stock prices of acquiring companies often drop after...
-
Q: Suggest one policy to improve the capital market of Bangladesh with justification. Q:Differentiate between the activities of Mutual Funds and Hedge Funds. Q:Differentiate between Foreign bonds and...

Study smarter with the SolutionInn App


