The vapor pressure of water at 25 C is 23.77 torr , increasing to 42.20 torr at
Question:
The vapor pressure of water at 25 °C is 23.77 torr , increasing to 42.20 torr at 35 °C . Calculate the standard free energy and standard enthalpy changes at 25 °C for the vaporization of water.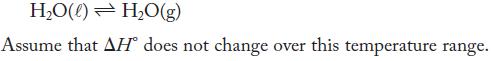
Strategy
First, determine the Gibbs free-energy change of the process; then use the Clausius–Clapeyron equation to determine the enthalpy change.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Because the equation includes the ratio of the two equilibrium constants it does not matter if we ex...View the full answer

Answered By

WAHIDUL HAQUE
hello,
I'm a professional academic solution provider working as a freelance academic solution provider since 7 years. I have completed numerous projects. Help lots of students to get good marks in their exams and quizzes. I can provide any type of academic help to your homework, classwork etc, if you are a student of Accounting, Finance, Economics, Statistics. I believe in satisfying client by my work quality, rather than making one-time profit. I charge reasonable so that we make good long term relationship. why will you choose me? i am an extremely passionate, boldly honest, ethically driven and pro-active contractor that holds each of my clients in high regards throughout all my business relations. in addition, I'll always make sure that I'm giving my 100% better in every work that will be entrusted to me to be able to produce an outcome that will meet my client's standards. so if you are a student that is now reading my profile and considering me for your academic help. please feel free to look through my working history, feedback and contact me if you see or read something that interests you. I appreciate your time and consideration.
regards
4.90+
233+ Reviews
368+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The vapor pressure of water at 25 C is 23.8 mmHg. Write K p for the vaporization of water, with pressures in atmospheres. What is the value of K c for the vaporization process?
-
(A) The reaction of aluminum with hydrochloric acid produces hydrogen gas. The balanced chemical equation for the reaction is given below. 2 Al(s) + 6 HCl(aq) 2 AlCl 3 (aq) + 3 H 2 (g) If 35.5 mL of...
-
You are a chemical engineer at a processing plant for soft drinks. You have been asked to determine whether a particular solution will have a significantly different vapor pressure from pure water....
-
Utility is a type of function that occurs in eco-nomics. When a consumer receives a units of a product, a certain amount of pleasure, or utility U, is gained. The following represents a typical...
-
N, N-Diethyl-m-toluamide (DEFT) is the active ingredient in many insect- repellent preparations. Flow might you synthesize this substance fromm-bromotoluene? . CH2CH3 N,N-Diethyl-m-toluamide CH-CH
-
Suppose the hypothetical processor of Figure 1.3 also has two I/O instructions: 0011 = Load AC from I/O 0111 = Store AC to I/O In these cases, the 12-bit address identifies a particular external...
-
The price of a share is \(\$ 100\). During the following six months the price can go up or down in a \(10 \%\) per month. If the risk-free interest rate is \(8 \%\) per year, continuously compounded,...
-
In 2013, Winslow International, Inc.s controller discovered that ending inventories for 2011 and 2012 were overstated by $200,000 and $500,000, respectively. Determine the effect of the errors on...
-
The Belfast router will perform NAT. Configure the Belfast router as follows: Define the NAT pool. The pool consists of public network address 200.10.10.64/26. Exclude first 10 addresses from this...
-
What is the work if a piston having an initial volume of 4.50 L contracts to 1.77 L against a constant external pressure of 1.34 atm? Is work done on the system or by the system?
-
The standard Gibbs free-energy change for the following reaction is +55.69 kJ . Strategy Because we know the G and the temperature, we can use Equation 17.11 to determine the equilibrium constant....
-
Explain the meaning of the quotation at the beginning of this chapter: Get the right data. Get the data right. Keep the data right.
-
Identify a recent economic, social, political, or technological trend that has significantly affected Facebook, and how the company was affecte?
-
Look at the social media marketing benefits listed above. Now look at the company's posts across platforms. Which of these marketing benefits do you think the company is gaining by posting on social...
-
The consensus sequence for Hpa II is 4 basepairs, while the consensus sequence for BsaBI is 12 basepairs. How frequently would each enzyme consensus site be predicted to be present in the human...
-
Earlier this fiscal year, Nugget Mining purchased a new piece of excavation equipment. How will this purchase affect Nugget's post-closing trial balance at the end of the fiscal year?
-
The accounting clerk for Smith Company mistakenly posted a closing entry to an expense account twice. How will this error affect the post-closing trial balance?
-
The COSO Internal Control, Integrated Framework describes an organization's internal controls as consisting of five elements. Required a. Briefly describe the relationship among the five components...
-
The following T-accounts show postings of selected transactions. Indicate the journal used in recording each of these postings a through e. Cash Accounts Receivable Inventory (d) 500 (e) 300 (b)...
-
Convert 125 ft 3 /s to gal/min.
-
Convert 0.060 ft 3 /s to gal/min.
-
Convert 7.50 ft 3 /s to gal/min.
-
How does a firm's external environment impact its strategic decision making process?
-
The following table lists the Actual cash flow for an investment. For the four years in question, the average inflation rate is 5% per year, the inflation free interest rate is 5% per year. Year 0 1...
-
Discuss the external environment scanning process, including the different types of information gathered during the scanning process and the importance of this information for strategic management.

Study smarter with the SolutionInn App


