Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a)
Question:
Use the Ka values in Table 15.6 to calculate the pH of the following solutions.
(a) 0.33 M HNO2
(b) 0.016 M phenol, C6H5OH
(c) 0.25 M HF
(d) 0.010 M HCOOH
Table 15.6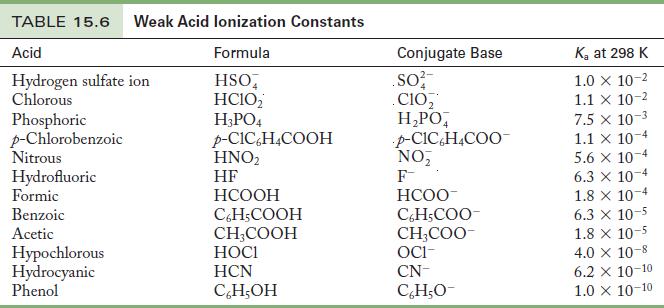
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 18...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
1 . Which of the following is a device --------------- a .water filter b. mouse c. air conditioning 2. is a unit of physical ---------------- hardware or equipment that provides one or more computing...
-
Consider a binomial distribution with 10 trials. Look at Table 2 in the Appendix showing binomial probabilities for various values of p, the probability of success on a single trial. (a) For what...
-
You are in the market for a new car and have narrowed your search down to two types: Car X costs $36,300, will last for four years, and will be worth $3,630 at the end of its useful life. It will...
-
A shell-and-tube heat exchanger with single shell and tube passes (Figure) is used to cool the oil of a large marine engine. Lake water (the shell-side fluid) enters the heat exchanger at 2 kg/s and...
-
A gas-turbine power plant operates on the simple Brayton cycle between the pressure limits of 100 and 1200 kPa. The working fluid is air, which enters the compressor at 30°C at a rate of 150...
-
Regularly attending the lectures of a course and passing that course. Determine whether the events are independent or dependent. Explain your reasoning.
-
The American Bakers Association reports that annual sales of bakery goods last year rose 15 percent, driven by a 50 percent increase in the demand for bran muffins. Most of the increase was...
-
Presented below is information related to the purchases of common stock by Nash Company during 2025. Cost Fair Value (at December 31) (at purchase date) Investment in Arroyo Company stock $90,000...
-
Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a) 0.050 M HI (b)0.85 M HF (c)0.15 M CH 3 COOH (d) 0.017 M C 6 H 5 COOH Table 15.6
-
Write the iCe table and set up the equation needed to solve for the concentration of the hydrogen ion in the following solutions. (a) 1.25 M HOCl (b)0.80 M HF (c)0.14 M CH3COOH (d) 0.25 M HCOOH
-
Sketch a throttling process of an ideal gas on a Ts diagram. Include the pressure contours that pass through the initial and final states.
-
As a project manager at Expedia, one of the largest online travel services in the world, youve seen plenty of college interns in action. However, few have impressed you as much as Maxine Max...
-
Why is it essential to understand your readers likely motivations before writing a persuasive message?
-
Your organization operates in an open and inclusive manner with regard to employee relations and interactions. For some years it has operated an employee suggestion scheme and has adopted some of the...
-
Claris Inc. is an advertising agency that is pitching for a new account at the baby food company Baby Bright. The agency has never held any previous account in this business and has no idea what the...
-
Discuss the comparative advantages and disadvantages of using emotional appeal and logical appeal.
-
Bergen Inc. produces telephone equipment at its Georgia plant. In recent years, the companys market share has been eroded by stiff competition from Asian and European competitors. Price and product...
-
Determine by direct integration the values of x for the two volumes obtained by passing a vertical cutting plane through the given shape of Fig. 5.21. The cutting plane is parallel to the base of the...
-
A BCD adder adds two BCD numbers (each of range 0 to 9) and produces the sum in BCD form. For example, if it adds 9 (1001) and 8 (1000) the result would be 17 (1 0111). Implement such a BCD adder...
-
Write synthesizable Verilog code that will generate the given waveform (W). Use a single always block. Assume that a clock with a 1 μs period is available as an input. -43 s -29 s+ -43...
-
(a) Implement the traffic-light controller of Figure 4-14 using a module 13 counter with added logic. The counter should increment every clock, with two exceptions. Use a ROM to generate the outputs....
-
The taxpayer should receive a full refund of $750 for the excess accumulation penalty they paid. Here's the explanation: Penalty Assessment: The IRS assessed a $750 penalty for not taking out the...
-
Piperel Lake Resort's four employees are paid monthly. Assume an income tax rate of 20%. Required: Complete the payroll register below for the month ended January 31, 2021. (Do not round intermediate...
-
1. Jody Solan currently insures her home for 100 percent of its replacement value with an HO-3 policy. For Jody, this works out to $189,000 in dwelling (Part A) coverage. What are the maximum dollar...

Study smarter with the SolutionInn App


