Use the standard reduction potentials in Table 18.1 to find (a) A reducing agent that will reduce
Question:
Use the standard reduction potentials in Table 18.1 to find
(a) A reducing agent that will reduce Cu2+ but not Pb2+.
(b) An oxidizing agent that will react with Cu but not Fe2+.
(c) A metal ion that can reduce Fe3+ to Fe2+.
Table 18.1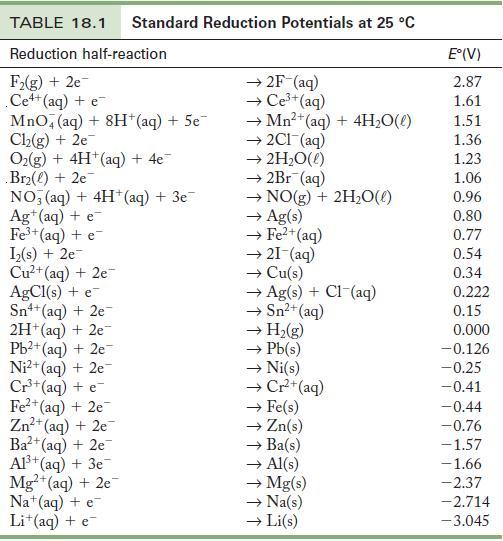
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a Keq Ni Cu2 ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.44 . Exercise 18.44 Use the standard reduction potentials in Table18.1 to find (a) A reducing agent that will...
-
Use the standard reduction potentials in Table 18.1 To find (a) A metal ion that reduces Ni 2+ . (b) A metal ion that can oxidize Cu. (c) A metal ion that is reduced by Cr 2+ but not H 2 . Table 18.1
-
Use the standard reduction potentials to find the equilibrium constant for each of the following reactions at 25°C: (a) (b) (c) Br2(1) + 21-(aq )- 2Br_(aq) + 12(s) 5Fe2 + (aq) +MnO4 (aq ) + 8H +...
-
Examples using activity-based costing generally show that traditional costing systems ________ high-volume, less complex products and ________ low-volume, complex products undercost; overcost...
-
An annular fin of thickness t is used as a radiator to dissipate heat for a space power system. The fin is insulated on the bottom and may be exposed to solar irradiation GS. The fin is coated with a...
-
Two CSMA/CD stations are each trying to transmit long (multi frame) files. After each frame is sent, they contend for the channel, using the binary exponential back off algorithm. What is the...
-
How many shims are too many under any one foot?
-
Your client, Schroeder Manufacturing Co., provided the following schedule of property, plant, and equipment for the year ended June 30, 2019. Balances have been agreed to the general ledger. As part...
-
Particle Q 1 = 3 . 2 mu C has a mass of 6 . 5 mu g , and particle Q 2 = 3 . 5 mu C has a mass of 7 . 6 mu g . The two - charges are initially separated by a distance of 2 . 4 cm . With charge Q 1...
-
Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.43 . Exercise 18.43 Use the standard reduction potentials in Table18.1 to find (a) A metal ion that reduces Ni...
-
Use the data in Appendix H and assume standard conditions when answering the following questions.
-
In Exercises 122133, use the strategy for solving word problems, modeling the verbal conditions of the problem with a linear inequality. To earn an A in a course, you must have a final average of at...
-
Why is it useful to integrate the collection of online and offline metrics?
-
Identify two examples of branding in financial services (e.g., specific types of retail bank accounts or insurance policies) and define their characteristics. How meaningful are these brands likely...
-
Describe four different types of site on which online display advertising for a car manufacturers site could be placed.
-
Visit the facilities of two competing service firms in the same industry (e.g., banks, restaurants, or gas stations) that you believe have different approaches to service. Compare and contrast their...
-
Compare the effectiveness of different methods of online advertising including display advertisements, paid search marketing and affiliate marketing.
-
Cherokee Corporation earned revenues of $37 million during 2012 and ended the year with net income of $7 million. During 2012, Cherokee collected cash of $20 million from customers and paid cash for...
-
As of January 1, 2018, Room Designs, Inc. had a balance of $9,900 in Cash, $3,500 in Common Stock, and $6,400 in Retained Earnings. These were the only accounts with balances in the ledger on January...
-
When 2000 L/min of water flows through a circular section with an inside diameter of 300 mm that later reduces to a 150-mm diameter, calculate the average velocity of flow in each section.
-
Figure 6.16 shows a fabricated assembly made from three different sizes of standard steel tubing listed in Appendix G.2. The larger tube on the left carries 0.072 m 3 /s of water. The tee branches...
-
A standard Schedule 40 steel pipe is to be selected to carry 10 gal/min of water with a maximum velocity of 1.0 ft/s. What size pipe should be used?
-
Assume you have been appointed to develop ethnic and multicultural marketing for a small chain of household appliance stores in large metropolitan area. There are several large concentrations of...
-
Pharoah Enterprises purchased a delivery truck on January 1 , 2 0 2 5 , at a cost of $ 2 6 , 0 0 0 . The truck has a useful life of 7 years with an estimated salvage value of $ 5 , 9 1 0 . The...
-
Consider the following recurrence relation: A(1)=1, A(n) = 2A(n-1)+2"-1 Use the method of unraveling to find a closed form for A(n).

Study smarter with the SolutionInn App


