Question: Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.43 . Exercise 18.43 Use the standard reduction potentials
Write an expression and determine a value for Keq for each voltaic cell in Exercise 18.43 .
Exercise 18.43
Use the standard reduction potentials in Table18.1 to find
(a) A metal ion that reduces Ni2+.
(b) A metal ion that can oxidize Cu.
(c) A metal ion that is reduced by Cr2+ but not H2.
Table 18.1 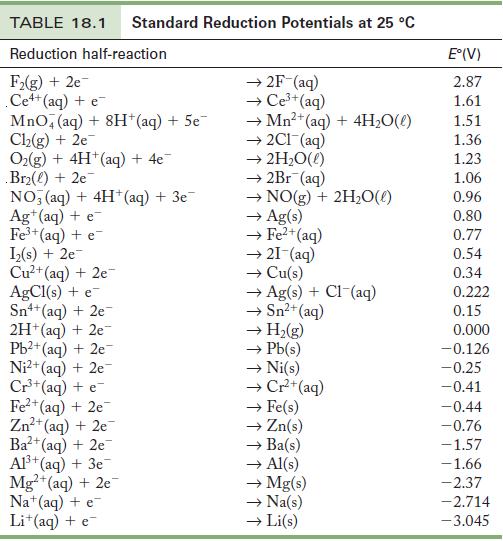
Step by Step Solution
3.34 Rating (163 Votes )
There are 3 Steps involved in it
To determine the value of the equilibrium constant Keq for a voltaic cell you can use the Nernst equ... View full answer

Get step-by-step solutions from verified subject matter experts


