Question: Using Table 9.4, calculate an approximate enthalpy change for (a) The reaction of molecular hydrogen (H 2 ) and molecular oxygen (O 2 ) in
Using Table 9.4, calculate an approximate enthalpy change for
(a) The reaction of molecular hydrogen (H2) and molecular oxygen (O2) in the gas phase to produce 2 mol water vapor.
(b) The reaction of carbon monoxide and molecular oxygen to form 2 mol carbon dioxide.
Table 9.4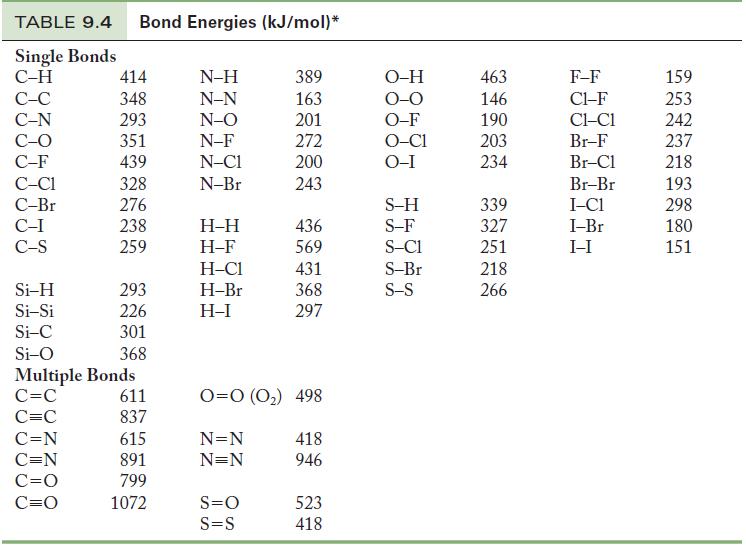
TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H C-C C-N C-O C-F C-C1 C-Br C-I C-S Si-H Si-Si Si-C Si-O 414 348 293 351 439 328 276 238 259 C=N C=N C=O C=O 293 226 301 368 Multiple Bonds C=C C=C 611 837 615 891 799 1072 N-H N-N N-O N-F N-C1 N-Br H-H H-F H-C1 H-Br H-I 389 163 201 272 200 243 S=O S=S 436 569 431 368 297 0=0 (0) 498 N=N N=N 418 946 523 418 O-H O-O O-F O-C1 O-I S-H S-F S-C1 S-Br S-S 463 146 190 203 234 339 327 251 218 266 F-F C1-F CLC1 Br-F Br-Cl Br-Br I-C1 I-Br I-I 159 253 242 237 218 193 298 180 151
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it
a 4... View full answer

Get step-by-step solutions from verified subject matter experts


