2H is a loosely bound isotope of hydrogen. Called deuterium or heavy hydrogen, it is stable but...
Question:
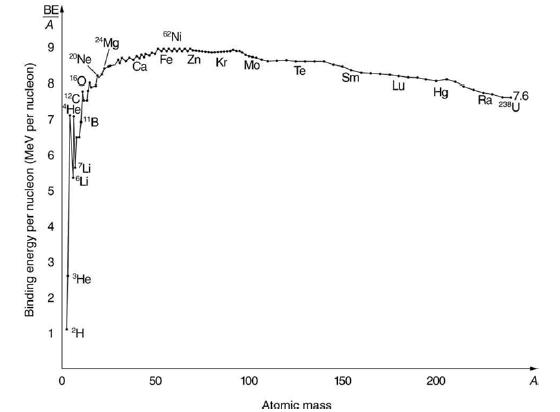
2H is a loosely bound isotope of hydrogen. Called deuterium or heavy hydrogen, it is stable but relatively rare-it is 0.015% of natural hydrogen. Note that deuterium has Z = N, which should tend to make it more tightly bound, but both are odd numbers. Calculate BE/A, the binding energy per nucleon, for 2H and compare it with the approximate value obtained from the graph in Figure 31.24.
Data from figure 31.24
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





