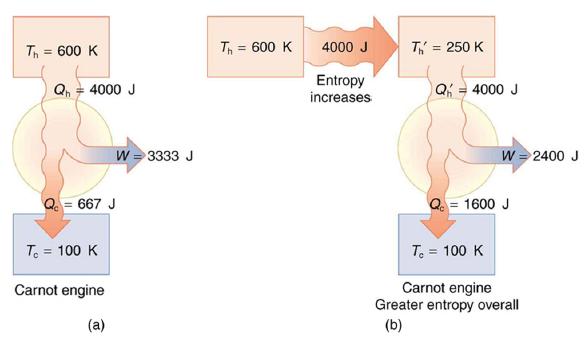
(a) Calculate the work output of a Carnot engine operating between temperatures of 600 K and 100...
Question:
(a) Calculate the work output of a Carnot engine operating between temperatures of 600 K and 100 K for 4000 J of heat transfer to the engine.
(b) Now suppose that the 4000 J of heat transfer occurs first from the 600 K reservoir to a 250 K reservoir (without doing any work, and this produces the increase in entropy calculated above) before transferring into a Carnot engine operating between 250 K and 100 K. What work output is produced? (See Figure 15.34.)
Strategy
In both parts, we must first calculate the Carnot efficiency and then the work output.
Data given in Figure 15.34
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





