Three ice cubes are used to chill a soda at 20C with mass m soda = 0.25
Question:
Three ice cubes are used to chill a soda at 20°C with mass msoda = 0.25 kg. The ice is at 0°C and each ice cube has a mass of 6.0 g. Assume that the soda is kept in a foam container so that heat loss can be ignored. Assume the soda has the same heat capacity as water. Find the final temperature when all ice has melted.
Strategy
The ice cubes are at the melting temperature of 0°C. Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two steps: first the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the temperature of this water rises. Melting yields water at 0°C, so more heat is transferred from the soda to this water until the water plus soda system reaches thermal equilibrium, Qice = -Qsoda
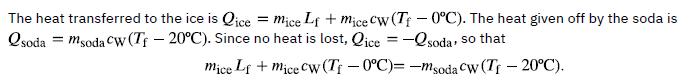
Bring all terms involving Tf on the left-hand-side and all other terms on the right-hand-side. Solve for the unknown quantity Tf:
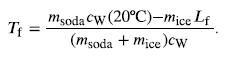
Step by Step Answer:






