Question: A water molecule (H 2 O) is shaped as shown in Figure P17.85A. The hydrogen?oxygen bond length is about 9.6 ? 10 -11 m, and
A water molecule (H2O) is shaped as shown in Figure P17.85A. The hydrogen?oxygen bond length is about 9.6 ? 10-11 m, and the bond angle is ? = 104?. To a first approximation, the molecule is composed of three point ions (two H+ ions and one O-2 ion).?
(a) What are the magnitude and direction of the electric field at the point midway between the O-2 ion and the line connecting the two H+ ions??
(b) We can model this system as the dipole in Figure P17.85B. If the electric field at the middle of this dipole is the same as your result in part (a), what is the effective charge Q??
(c) Use the effective dipole model to calculate the force between two water molecules that are separated by a distance of 0.30 nm (the typical separation in liquid water). Assume the dipoles of the two water molecules are oriented in opposite directions with their axes perpendicular to the line connecting the centers of the dipoles.
Figure P17.85
?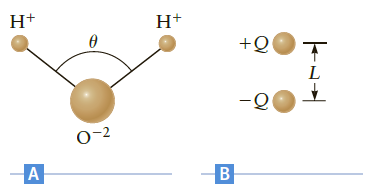
H+ +Q L - 0-2 A B
Step by Step Solution
3.37 Rating (172 Votes )
There are 3 Steps involved in it
a The electric field at the center of the triangle is the vector sum of the fields due to each of the three ions individually The magnitude of the ele... View full answer

Get step-by-step solutions from verified subject matter experts


