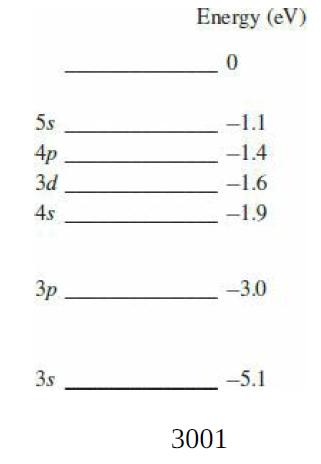
The figure shows the lowest six energy levels of the outer electron in sodium. In the ground
Question:
The figure shows the lowest six energy levels of the outer electron in sodium. In the ground state, the electron is in the “3s” level.
(a) What is the ionization energy of sodium?
(b) What is the wavelength of the radiation emitted in the transition from the 3d to the 3p level?
(c) What is the transition that gives rise to the characteristic yellow light of sodium at 589 nm?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:





