A series of solutions containing NaOH, Na 3 AsO 4 , and Na 2 HAsO 4 ,
Question:
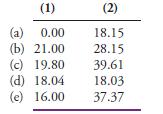
A series of solutions containing NaOH, Na3AsO4, and Na2HAsO4, alone or in compatible combination, was titrated with 0.08601 M HCl. In the following table are the volumes of acid needed to titrate 25.00- mL portions of each solution to (1) a phenolphthalein and (2) a bromocresol green end point. Use this information to deduce the composition of the solutions. In addition, calculate the mass in milligrams of each solute per milliliter of solution.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:





