Potassium can be determined by flame emission spectrometry (flame photometry) using a lithium internal standard. The following
Question:
Potassium can be determined by flame emission spectrometry (flame photometry) using a lithium internal standard. The following data were obtained for standard solutions of KCl and an unknown containing a constant, known amount of LiCl as the internal standard. All the intensities were corrected for background by subtracting the intensity of a blank.
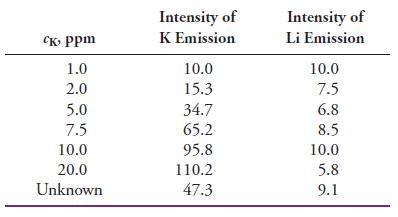
(a) Plot the K emission intensity versus the concentration of K, and determine the linearity from the regression statistics.
(b) Plot the ratio of the K intensity to the Li intensity versus the concentration of K, and compare the resulting linearity to that in part (a). Why does the internal standard improve linearity?
(c) Calculate the concentration of K in the unknown.
Step by Step Answer:

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch





