An ideal gas has C P *=(7/2)R. One mole of this gas is confined in a piston-cylinder
Question:
An ideal gas has CP*=(7/2)R. One mole of this gas is confined in a piston-cylinder device. Initially, the gas is at T = 300 K and P = 1 bar (Figure 3-13). If the gas is compressed isothermally to P = 5 bar, find the amounts of work and heat associated with the process.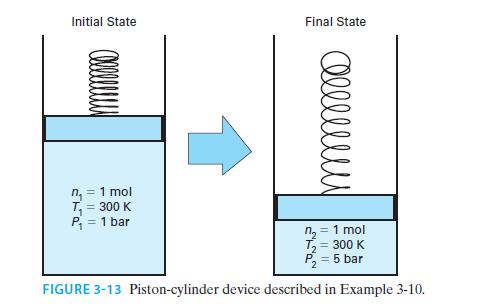
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Step 1 Define the system Define the system as the gas inside the cylinder Step 2 Apply and simplify ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
A fundamental aspect of Six Sigma methodology is the identification of critical to quality (CTQ) characteristics that are vital to customer satisfaction. Identify and depict the various activities...
-
Refer to M3-5 for information regarding Stone Company. Using the FIFO method, reconcile the number of physical units and calculate the number of equivalentunits. Physical Units 70,000 92,000 Percent...
-
James came back from Overseas to seek full-time employment in Singapore. He has the following stock options which were granted to him in 2021 and 2022: In 2021, a stock option for 10,000 shares at an...
-
Finally, Reza mentions that he was hired into the CFO role on an interim basis with the possibility of being made permanent based on performance. Although he wants to succeed in this role, he does...
-
Consider Review Exercise 9.101. Let us assume that the data have not been collected yet. Let us also assume that previous statistics suggest that 1 = 2 = $4000. Are the sample sizes in Review...
-
Presented below is the comparative balance sheet for Diatessaron Inc., a private company reporting under ASPE, at December 31, 2017 and 2016: Additional information: 1. Cash dividends of $15,000 were...
-
Nereus Montemayor was an employee of VZ Hogs, a company that raises hogs and produces hog feed. VZ Hogs used an extruder manufactured by Sebright Products, Inc. to create hog feed out of discarded...
-
Mathews Guitar Company makes high-quality customized guitars. Mathews uses a job order costing system. Because the guitars are handmade, the company applies overhead based on direct labor hours. At...
-
5. WORK HEALTH & SAFETY Provide at least three types of hazards, corresponding risks, rating and control measures. Risk Rating (Low/Medium/High) Control measures (Current/Required)
-
The balloon portion of a dirigible contains 5000 moles of helium. Initially, the helium is at T = 15C and P = 0.95 atm, and is 500 m off the ground (where the atmospheric pressure is also 0.95 atm)....
-
A tank has a volume of 500 liters and is initially full of a liquid that is at 300 K, has a density of 0.8 kg/L, and a constant heat capacity of C V = 3 J/g K. The tank can be modeled as perfectly...
-
Chapter 5 states that total spending always equals total output in national income accounting. So how can aggregate demand be different from aggregate supply in this chapter?
-
Questions: 1) Summarize chapter 3 2) What is disaster recovery? Explain short-term recovery and long-term recovery. 3) How does recovery start? Preliminary damage assessment (PDA). 4) Hoe does...
-
Consider the following assembly code generated for a function int F(int x, int y): wwww push mov www ebp ebp esp sub esp, 4 mov wwwwww mov wwwwww add mov wwww mov www sub mov www leave DWORD PTR...
-
Elizabeth, a resident, carries on a retail business selling health foods to the public. Up to 30 April o the current tax year she operated her business from her own premises. On 30 April she sold her...
-
Write a Matlab program to accept a string as input and determine if this string is a palindrome. A palindrome is a sentence that reads the same backward as forward, such as "Reward a Toyota drawer"...
-
Aleksandr Global Corporation wants to create a sales budget. The company anticipates the projected following sales per quarter: Quarter 1 = 12,000 units, Quarter 2 = 15,000 units, Quarter 3 = 10,000...
-
Why do corporations utilize different forms of equity?
-
Show that every group G with identity e and such that x * x = e for all x G is abelian.
-
Represent each of the following combinations of unit in the correct SI form: (a) Mg/ms, (b) N/mm, (c) mN/(kgs).
-
Convert: (a) 200 lb ft to N.m (b) 350 lb/ft 3 to kN/m 3 (c) Express the result to three significant figures. Use an appropriate prefix.
-
Represent each of the following as a number between 0.1 and 1000 using an appropriate prefix: (a) 45320 kN, (b) 568(105) mm, (c) 0.00563 mg.
-
Delaware Chemical Company uses oil to produce two types of plastic products, P1 and P2. Delaware budgeted 24,400 barrels of oil for purchase in June for $71 per barrel. Direct labor budgeted in the...
-
Think about organizations that you have been involved in either in the past or currently. Can you remember their slogan? Why would remembering it be valuable for organizations?
-
Income tax expense Net income (17,800) (10,000) $26,700 $15,000 a. Prepare a comparative income statement with horizontal analysis, indicating the increase (decrease) for the current year when...

Study smarter with the SolutionInn App


