An ideal gas has C P * = (7/2)R. We are designing a steady-state process to compress
Question:
An ideal gas has CP* = (7/2)R. We are designing a steady-state process to compress the gas from an initial state of P = 1 bar and T = 300 K to a final state of P = 64 bar and T = 135 K. Find the work added and heat removed per mole of gas for each of the following processes (summarized in Figure 5-16).
A. The gas is compressed to P = 64 bar in an adiabatic, reversible compressor and then enters a heat exchanger in which it is cooled to T = 135 K.
B. The gas is cooled to T = 135 K in a heat exchanger, compressed to P = 64 bar in an adiabatic reversible compressor, and in a second heat exchanger, is cooled to T=135 K.
C. The gas is compressed in two separate adiabatic, reversible compressors. The first has inlet P = 1 bar and outlet P = 8 bar, and the second compresses the gas from P = 8 bar to P = 64 bar. Each compressor is preceded by a heat exchanger that cools the gas to T = 135 K, and a third heat exchanger cools the final product to T= 135 K.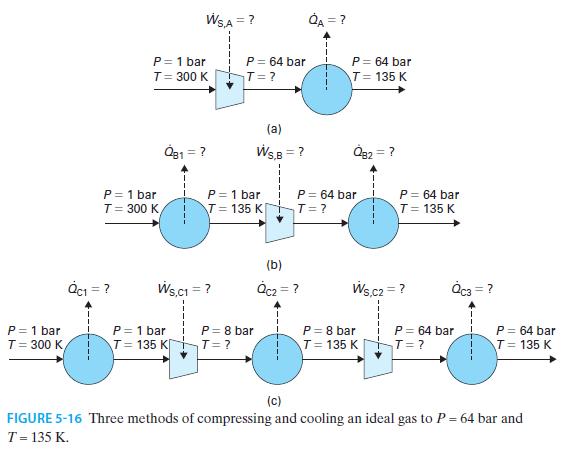
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





