Chromatography is a process by which a separation of chemical species is accomplished by selective adsorption on
Question:
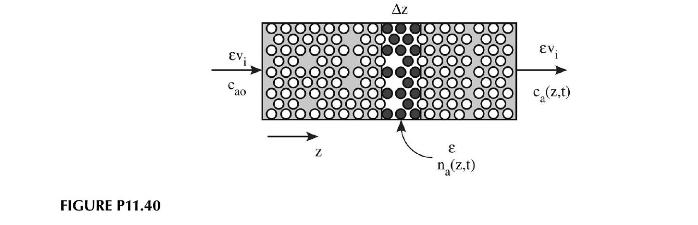
Chromatography is a process by which a separation of chemical species is accomplished by selective adsorption on a solid medium. Consider the simple case of a packed column of cross-sectional area, \(A_{c}\) and length, \(L\) as shown in Figure P11.40. The void fraction (liquid space) within the column is \(\varepsilon\). Fluid containing the adsorbate in a concentration of \(c_{a o}\) (moles adsorbate \(/ \mathrm{m}^{3}\) fluid), is introduced to the column at a velocity, \(v_{i}\), characteristic of the fluid velocity in the interstices between adsorbent particles. The concentration of adsorbate on the particles themselves is \(c_{p a}\) (moles of adsorbate \(/ \mathrm{m}^{3}\) of adsorbent). By performing a balance about a small slice through the column, show that the differential equation governing the concentration of adsorbate in the fluid is governed by the following equation:
\[\varepsilon v_{i} \frac{\partial c_{a}}{\partial z}+\varepsilon \frac{\partial c_{a}}{\partial t}+(1-\varepsilon) \frac{\partial c_{p a}}{\partial t}=0\]
where we use the relation:
\[(1-\varepsilon) \frac{\partial c_{p a}}{\partial t}=f\left(c_{a}, c_{p a}, v_{i}, \varepsilon, \ldots\right)\]
to describe the finite rate of transfer of adsorbate from solute to adsorbent. It takes the place of \(k A \Delta c\) to describe the mass transfer rate.
Step by Step Answer:






