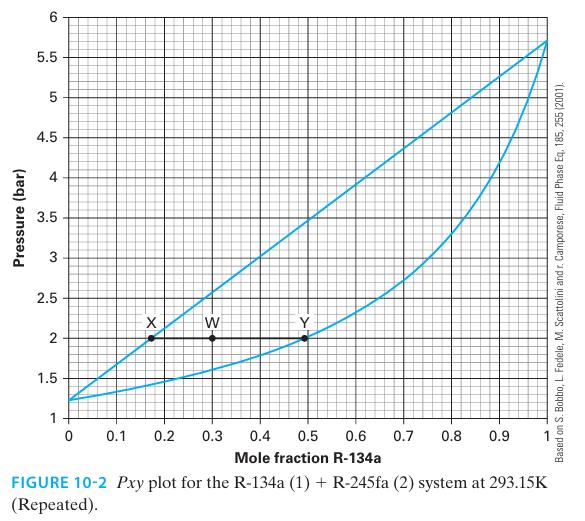
If we mix 3 moles of R-134a (1) and 7 moles of R-245fa (2) together at 293
Question:
If we mix 3 moles of R-134a (1) and 7 moles of R-245fa (2) together at 293 K and 2.0 bar, what are the composition and amounts of the phase(s) present at equilibrium? Use the lever rule.
Transcribed Image Text:
Pressure (bar) 6 5.5 LO 5 4.5 4 3.5 2.5 2+ 1.5 1 X 0 0.1 0.2 -3- W > 0.3 0.4 0.5 0.6 0.7 0.8 0.9 Mole fraction R-134a FIGURE 10-2 Pxy plot for the R-134a (1) + R-245fa (2) system at 293.15K (Repeated). Based on S. Bobbo, L. Fedele, M. Scattolini and r. Camporese, Fluid Phase Eq. 185, 255 (2001).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Where is the point on the mixture phase diagram Liquid vapor or both Fortunately we have a phase dia...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Suppose the economy is initially at point A in Exhibit 25-1. If government purchases increase, which point best depicts where the economy will be in the short run as a result of the change in...
-
Kellam Images prints snack bags on long rolls of plastic film. The plant operates 250 days a year. The daily production rate is 6,000 bags, and the daily demand is 3,500 bags sold for $2 each. The...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
(a) Show that (x) = 2x + 3x 36x is not one-to-one on (-, ). (b) Determine the greatest value c such that is one-to-one on (-c, c).
-
Contrast the two types of project life cycles and discuss why it is important to know which type the current project is following.
-
Assume the same information as in E14-4, except that the bonds were issued at 84.95 to yield 12%. Instructions Prepare the journal entries to record the following. (Round to the nearest euro.) (a)...
-
Reconsider the data from Problem 56. What is the capital recovery cost of Model 334A? Data from problem 56 Octavia Bakery is planning to purchase one of two ovens. The expected cash flows for each...
-
Tom Belford and Tony Sorrentino own a small business devoted to kitchen and bath granite installations. Recently, building contractors have insisted on up-front bid prices for a house rather than the...
-
Thunderduck Shoes provides shoe shining and repair services to customers. For the year which ended Dec 31, the company reports the following amounts: Account Amount Account Amount Rent Expense 22,400...
-
Does an equimolar mixture of methanol (1) + acetone (2) exist as one or two phases at 101.325 kPa and 334 K? Plot the entire Txy curve to find out. Use Raoults Law to estimate the phase coexistence.
-
What is the entropy change when you mix one mole of butane gas with one mole of propane gas, modeled as an ideal gas mixture? Assume the pressure and temperature of the system are constant during...
-
Reread the Management Focus on Philips in China and then answer the following questions: a. What are the major benefits to Philips of shifting so much of its global production to China? b. What are...
-
A small piece of plastic is given a net charge of + 1.17 C. How many more protons than electrons does the piece of plastic now have? (e = 1.602x10-19 C and 1 C = 1.00x10-6 C)
-
If WiseGuy Inc. uses the IRR rule to choose projects, which of the projects will rank highest?
-
The market has the following probability distributions: Probability market 0.3 12% 0.4 4% 0.3 24 Calculate the standard deviation for the market. Explain and how to solve on BA II Plus financial...
-
Ghost Corporation was owned 100% by Caspar. Upon Caspar's death, Ghost was worth $10,000,000. Prior to his death, Caspar's basis in his Ghost Corporation stock was $1,000,000. Caspar's total estate...
-
JPL incurred the following costs relating to an overseas promotional and advertising campaign for the year ended 31 December 2022: $ Stand rental 90,000 Corporate brochures 20,000 Airfares for three...
-
As the manager of Smith Construction, you need to make a decision on the number of homes to build in a new residential area where you are the only builder. Unfortunately, you must build the homes...
-
we have to compute the letter grades for a course. The data is a collection of student records stored in a file. Each record consists of a name(up to 20 characters), ID (8 characters), the scores of...
-
The differential equation model for a certain position control system for a metal cutting tool is where the actual tool position is x; the desired position is x d (t); and K p , K I , and K D are...
-
The differential equation model for a certain speed control system for a vehicle is where the actual speed is , the desired speed is d (t), and K p and K I are constants called the control gains....
-
The differential equation model for the motor torque m(t) required for a certain speed control system is where the desired speed is !d(t) and K is a constant called the control gain. a. Use the...
-
the fewer the linkages between the businesses within the portfolio owned by the firm
-
What is the exception principle of control? Give some examples of exception principle of control. How it works.
-
Greenlife Inc. is a CCPC. For the taxation year ending December 31, 2023, its taxable income of $140,000 is made up of active business income of $50,000 and investment income of $55,000, all of which...

Study smarter with the SolutionInn App


