In a process analysis application, you are working with the di-n-propyl ether (1) and 2-propanol (2) system
Question:
In a process analysis application, you are working with the di-n-propyl ether (1) and 2-propanol (2) system at 25°C. You think you have an error in the spread sheet you have been working with, but you can’t seem to find the problem. After a while, you begin to wonder if the data you are using is thermodynamically consistent. Not that you are suspicious of the data, but you’ve checked everything else by this point.

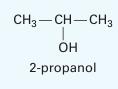
For the data presented in Table P11-29, examine the thermodynamic consistency using both the integral test and direct test.
![TABLE P11-29 Vapor-liquid equilibrium of di-n-propyl ether (1) + 2-propanol (2) at 298.15 K. P[kPa] 2.878](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/3/8/8/817653b5b51aff741698388815985.jpg)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





