In Problem 12-18 in this section, you used a gamma-phi modeling approach for the pentafluoroethane [R-125] (1)
Question:
In Problem 12-18 in this section, you used a gamma-phi modeling approach for the pentafluoroethane [R-125] (1) + isobutane (2) system at 30°C. There (if you solved that problem), you realized the benefit of incorporating a gamma-phi approach (i.e., treating the vapor phase as a real gas rather than an ideal gas) as compared to using modified Raoult’s law.
In this problem repeat the gamma-phi modeling, but treat the vapor-phase as an ideal solution. Here, you are not including the composition effects on the fugacity coefficient, but modeling it as a pure component at the mixture temperature and pressure. Plot both results (the full gamma phi approach from Problem 12-18 and the cur rent approach) as well as the experimental data (as symbols). Additionally, report the following information in tabular form:
∎ The experimental activity coefficients for both approaches
∎ The ratio of the mixture fugacity coefficient of component i to the saturation fugacity coefficient of component i
What can you conclude about the ideal solution approach to the vapor phase in the context of this problem?
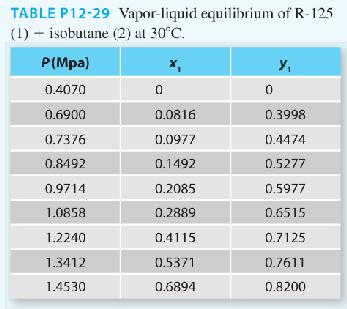
Problem 12-18.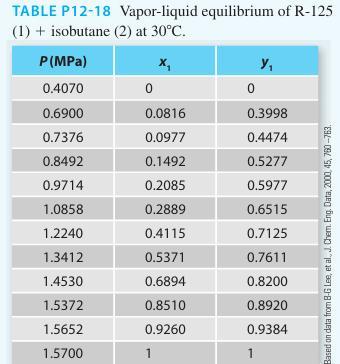
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco




