The solubility product constant for magnesium hydroxide, (operatorname{Mg}(mathrm{OH})_{2}), in water is (K_{s p}=1.2 times 10^{-11}(mathrm{kmol} / mathrm{m})^{3}).
Question:
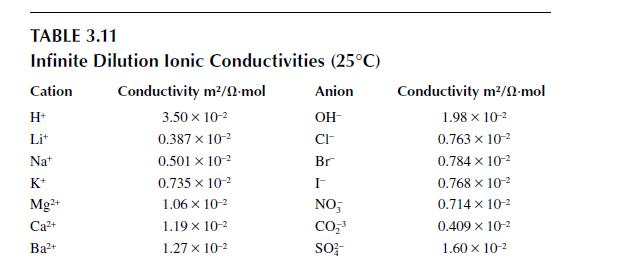
The solubility product constant for magnesium hydroxide, \(\operatorname{Mg}(\mathrm{OH})_{2}\), in water is \(K_{s p}=1.2 \times 10^{-11}(\mathrm{kmol} / \mathrm{m})^{3}\). If \(5 \mathrm{~g}\) of magnesium hydroxide were put into \(50 \mathrm{ml}\) of water and allowed to dissolve to equilibrium, estimate the molar conductivity of the solution at \(298 \mathrm{~K}\). (The mobility and diffusivity of \(\mathrm{Mg}^{2+}\) are not given in the tables. However, data for \(\mathrm{Ca}^{2+}\) exist in Table 3.12 and data for the infinite dilution conductivity of a Mg-based and Ca-based solution exist in Table 3.11. Based on that information, what is your best estimate for the mobility and diffusivity of \(\mathrm{Mg}^{2+}\) ?)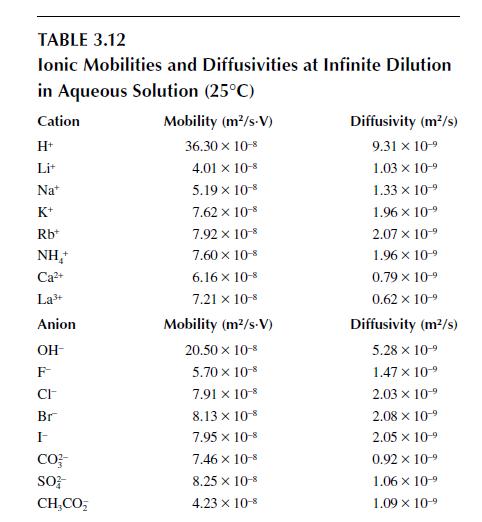
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





