(A) At room temperature (20C), milk turns sour in about 64 hours. In a refrigerator at 3C,...
Question:
(A) At room temperature (20°C), milk turns sour in about 64 hours. In a refrigerator at 3°C, milk can be stored three times as long before it sours.
(a) Estimate the activation energy of the reaction that causes the souring of milk.
(b) How long should it take milk to sour at 40°C?
(B) The following mechanism can be used to account for the change in apparent order of unimolecular reactions, such as the conversion of cyclopropane (A) into propene (P), where A* is an energetic form of cyclopropane that can either react or return to unreacted cyclopropane.
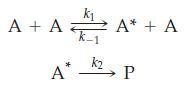
Show that at low pressures of cyclopropane, the rate law is second order in A and at high pressures, it is first order in a.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





