Question: (A) Equilibrium is established between liquid hexane, C 6 H 14 , and its vapor at 25.0 C. A sample of the vapor is found
(A) Equilibrium is established between liquid hexane, C6H14, and its vapor at 25.0 °C. A sample of the vapor is found to have a density of 0.701 g/L. Calculate the vapor pressure of hexane at 25.0 °C, expressed in Torr.
(B) With the help of Figure 12-18, estimate the density of the vapor in equilibrium with liquid diethyl ether at 20.0 °C.
Figure 12-18
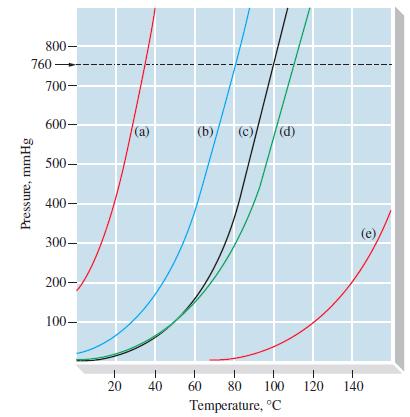
800- 760 Pressure, mmHg 700- 600- 500 400- 300- 200- 100- **** 20 (a) 1 40 (b) (c) (d) I 60 1 1 80 100 120 Temperature, C 1 140
Step by Step Solution
3.30 Rating (162 Votes )
There are 3 Steps involved in it
A To calculate the vapor pressure of hexane at 250 C we can use the ideal gas law PV nRT where P is ... View full answer

Get step-by-step solutions from verified subject matter experts


