(A) If the reaction described in this example resulted in H 2 O produced and maintained at...
Question:
(A) If the reaction described in this example resulted in H2O produced and maintained at would the water be present as vapor only or as liquid and vapor in equilibrium? Explain.
(B) For the situation described in Example 12.4, what mass of water is present as liquid and what mass as vapor?
Example 12.4
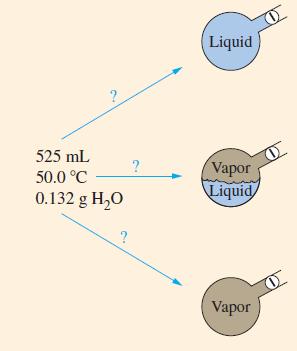
As a result of a chemical reaction, 0.132 g H2O is produced and maintained at a temperature of 50.0 °C in a closed flask of 525 mL volume. Will the water be present as liquid only, vapor only, or liquid and vapor in equilibrium (Fig. 12-19)?
Figure. 12-19
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





