A tabulation of more precise thermodynamic data than are presented in Appendix D lists the following values
Question:
A tabulation of more precise thermodynamic data than are presented in Appendix D lists the following values for H2O(l) and H2O(g) at 298.15 K, at a standard state pressure of 1 bar.
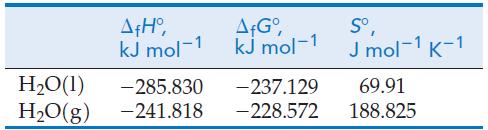
(a) Use these data to determine, in two different ways, ΔrG° at 298.15 K for the vaporization: H2O (l, 1 bar) ⇌ H2O(g, 1 bar).
(b) Use the result of part (a) to obtain the value of K for this vaporization and, hence, the vapor pressure of water at 298.15 K.
(c) The vapor pressure in part (b) is in the unit bar. Convert the pressure to millimeters of mercury.
(d) Start with the value ΔrG° = 8.590 kJ mol-1, and calculate the vapor pressure of water at 298.15 K in a fashion similar to that in parts (b) and (c). In this way, demonstrate that the results obtained in a thermodynamic calculation do not depend on the convention we choose for the standard state pressure, as long as we use standard state thermodynamic data consistent with that choice.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





