(A) Which of the four cases in Table 13.3 would apply to each of the following reactions?...
Question:
(A) Which of the four cases in Table 13.3 would apply to each of the following reactions?
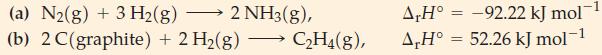
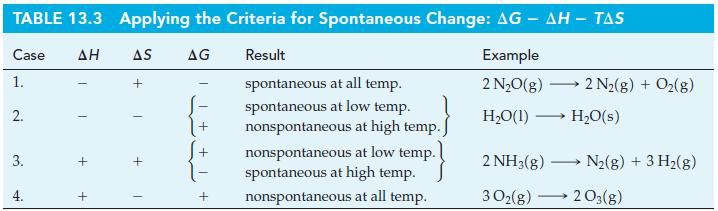
(B) Under what temperature conditions would the following reactions occur spontaneously?
(a) The decomposition of calcium carbonate into calcium oxide and carbon dioxide. The reaction is endothermic.
(b) The “roasting” of zinc sulfide in oxygen to form zinc oxide and sulfur dioxide. This exothermic reaction releases 439.1 kJ for every mole of zinc sulfide that reacts.
Table 13.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





