Arrange the entropy changes of the following processes, all at 25 C, in the expected order of
Question:
Arrange the entropy changes of the following processes, all at 25 °C, in the expected order of increasing ΔS, and explain your reasoning:
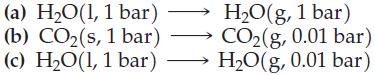
Transcribed Image Text:
(a) H₂O(1, 1 bar) (b) CO₂ (s, 1 bar) (c) H₂O(1, 1 bar) 111 H₂O(g, 1 bar) CO₂(g, 0.01 bar) H₂O(g, 0.01 bar)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Process Entropy change S Explanation a H2Ol 1 bar H2Og 1 bar 4605 JKmol This is the vaporization of ...View the full answer

Answered By

Jayshree Rathi
Hello Students!
This is Jayshree Rathi. I work on a number of renowned student-centric channels such as Chegg, coursehero, as a certified private tutor.
If you are looking for relevant and original content to complete your assignments, essays, and homework, then contact me and within the promised time, I will deliver you your personalized academic work and help you score the best.
4.80+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Justin Bieber has emerged as a bona fide sports star, but in the most unlikely of sportstable tennis. At only 24 years old, Bieber has won 8 of the last 11 major table tennis tournaments over the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Mr Brooks, a local builder specialized in multi-story buildings, made this arrangement to convert his construction company into a trust after his death. Mr Tim, who was the manager for this...
-
For rigid-body motion, the strains will vanish. Under these conditions, integrate the straindisplacement relations (7.6.1) to show that the most general form of a rigid-body motion displacement field...
-
Garza and Neely, CPAs, are preparing their service revenue (sales) budget for the coming year (2014). The practice is divided into three departments: auditing, tax, and consulting. Billable hours for...
-
Draw the shear and moment diagrams for thebeam. 1/2 AT
-
Several professionals are listed as being part of compliance efforts with regard to participation in the examination of potential compliance violations. Identify at least three other categories of...
-
Vulcan Companys contribution format income statement for June is given below: Management is disappointed with the companys performance and is wondering what can be done to improve profits. By...
-
Thunderduck Shoes provides shoe shining and repair services to customers. For the year which ended Dec 31, the company reports the following amounts: Account Amount Account Amount Rent Expense 22,400...
-
(A) Which of the four cases in Table 13.3 would apply to each of the following reactions? (B) Under what temperature conditions would the following reactions occur spontaneously? (a) The...
-
Under what temperature conditions would the following reactions occur spontaneously? (a) 2 NH4NO3(s) - (b) I2(g) 2 I(g) 2 N(g) + 4 HO(g) + O2(g) A,H -236.0 kJ mol- =
-
As indicated in the chapter, the CBO forecast that real GDP would grow at an average annual rate of 1.9 percent from 2017 to 2027. The Trump administration pledged to raise the growth rate to 3...
-
Describe the classical method to determine probability with example.
-
Outline the key features of each of the seven steps in the quantitative analysis approach.
-
In a scatter diagram, the independent variable is plotted on the a. vertical axis. b. trend line. c. horizontal axis. d. origin of the axis.
-
What are the strengths of using decision trees?
-
Explain the limitation in the use of graphical solution for an LP problem. What are its advantages, even when its specific application is not suitable?
-
Microtech Incorporated has decided to package its cell phone in a smaller, recyclable package. Additionally, the company will discontinue the practice of shipping each phone with a 250-page user...
-
The domain of the variable in the expression x 3/x + 4 is________.
-
Supply chain effects on total relevant inventory cost. Cow Spot Computer Co. outsources the production of motherboards for its computers. It has narrowed down its choice of suppliers to two...
-
Back flush costing and JIT production. The Acton Corporation manufactures electrical meters. For August there were no beginning inventories of direct materials and no beginning or ending work in...
-
Backflush, two trigger points, materials purchase and sale (continuation of 20-33). Assume that the second trigger point for Acton Corporation is the salerather than the completionof finished goods....
-
1- Create a new script that is called HW11a.m and save it. The script will do the following: a. Evaluate the following sum using a for loop: 10 x = n n-1 b. Display the value of x using disp command...
-
2. (8 points) Consider the following list of nouns and noun phrases: address make cars Anthony's car year Porsche total estimated BMW service quote sales tax John's class model charges estimated...
-
For the system shown below, find the transfer function assuming that the output is y and the input is x X(s) 25 S + 1 1 10 3 4 S Y(s)

Study smarter with the SolutionInn App


