Question: Ascorbic acid (C 6 H 8 O 6 ), also commonly known as vitamin C, can be used to reduce a wide variety of transition
Ascorbic acid (C6H8O6), also commonly known as vitamin C, can be used to reduce a wide variety of transition metal ions. Given that E°cell = 0.71 V for the reaction
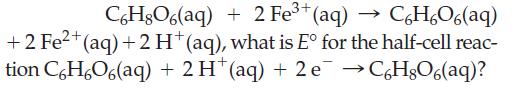
C6H8O6(aq) + 2 Fe+ (aq) >>> C6HO6(aq) +2 Fe+ (aq) + 2 H+ (aq), what is E for the half-cell reac- tion C6HO6(aq) + 2 H (aq) + 2 e HgO6(aq)?
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it
To determine the standard reduction potential E for the halfcell reaction C6H6O6aq 2Haq 2e ... View full answer

Get step-by-step solutions from verified subject matter experts


