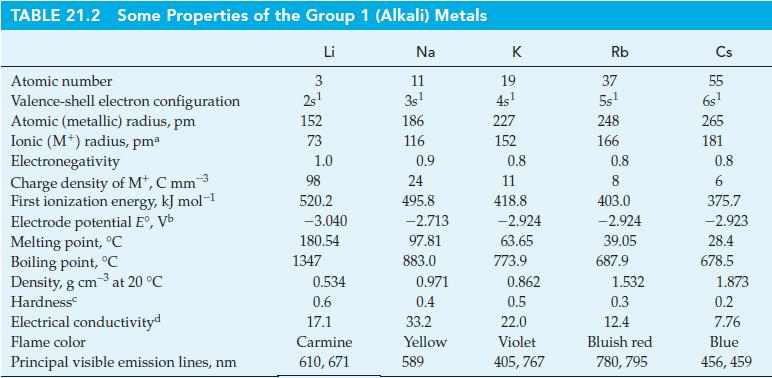
Comment on the feasibility of using a reaction similar to (21.4) to produce (a) Lithium metal from
Question:
Comment on the feasibility of using a reaction similar to (21.4) to produce
(a) Lithium metal from LiCl;
(b) Cesium metal from CsCl, with Na(l) as the reducing agent in each case. Consider data from Table 21.2.
Reaction (21.4)
![]() Table 21.2
Table 21.2
Transcribed Image Text:
KCI(1) + Na(1) 850 °C NaCl(1) + K(g) (21.4)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
KCl Na a LiCl Na NaCl K NaCl Li Comments It is not feasible ...View the full answer

Answered By

RADHIKA MEENAKAR
I am a qualified indian Company Secretary along with Masters in finance with over 6 plus years of professional experience. Apart from this i am a certified accounts and finance tutor on many online platforms.
My Linkedin profile link is here https://www.linkedin.com/in/radhika-meenakar-88b9808a/
5.00+
12+ Reviews
22+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Digital Report Question 1: Find a brand of your choice that you believe it has successfully used social publishing, explain how the brand has effectively used social publishing in relation to the...
-
Given the plant design an integral controller to yield a 15% overshoot, 0.6-second settling time, and zero steady-state error for a step input. -1 1] u; y= [1 1]x X+ 0 2
-
Identify the process evaluation article that you chose and explain why you selected this example. Describe the purpose of the evaluation, the informants, the questions asked, and the results of the...
-
Simplify the expression 2x + (x + 1) into a single x + 1 fraction. The numerator of your answer is: The denominator of your answer is:
-
The post-closing trial balance of Anderson Company at December 31, 2011, is shown here. During 2012, Anderson Company had the following transactions: a. Inventory purchases were $80,000, all on...
-
Regulators have traditionally required banks to maintain capital-asset ratios of a certain level to ensure adequate net worth based on the size and composition of the banks asset on its balance...
-
Xie Company identified the following activities, costs, and activity drivers for 2017. The company manufactures two types of go-karts: deluxe and basic. Required 1. Compute a single plantwide...
-
The following probabilistic activity time estimates are for the CPM/PERT network in Problem Determine the following: a. Expected activity times b. Earliest start and finish times c. Latest starts and...
-
Consider the Solow model we studied in class, with the production function given by: Y =AKN1. tttt In this question, we distinguish between the labor force (L) and the total population (Pop). The...
-
Concerning the thermite reaction, (a) Use data from Appendix D to calculate r H at 298 K for the reaction below. (b) Write an equation for the reaction when MnO 2 (s) is substituted for Fe 2 O 3...
-
When a 0.250 g sample of Ca is heated in air, 0.325 g of product is obtained. Assume that all the Ca appears in the product. (a) If the product were pure CaO, what mass should have been obtained? (b)...
-
Prescription drugs obtained from sources outside the United States, such as Canada, are a. Always deductible no matter how they were obtained. b. Deductible only for citizens of Canada living in the...
-
Small businesses often need to come up with a unique angle to attract any kind of media attention. Sizzle and Grill in Cardiff, South Wales, United Kingdom, was the home to 40 different mega-meals....
-
Using the questions in Figure 4.3, write a brief analysis of the audience for each of the following communication tasks. What kind of reaction should you expect from the primary reader and any...
-
Although the merits and problems of breast-feeding versus using infant formula are debated in the United States and other developed countries, the issue is not so balanced in third-world nations....
-
Once the Consumer Product Safety Commission prohibits the sale of a particular product in the United States, a manufacturer can no longer sell the product to U.S. wholesalers or retailers. However,...
-
The global market presents firms with more complex ethical issues than they would experience if operations were limited to one country and one culture. Moral standards vary across cultures. In some...
-
Chet is an officer of the Branson Corporation, a publicly traded corporation. His salary for the year is $1,320,000, which is the sixth-highest salary at Branson. What amount can the corporation...
-
Prairie Outfitters, Inc., a retailer, accepts paymnent through credit cards. During August, credit card sales amounted to $12,000. The processor charges a 3% fee. Assuming that the credit card...
-
(a) The steps in the accounting cycle for a merchandising company differ from the steps in the accounting cycle for a service company. Do you agree or disagree? (b) Is the measurement of net income...
-
How do the components of revenues and expenses differ between a merchandising company and a service company?
-
Rachel Harpole, CEO of Bargain Den Stores, is considering a recommendation made by both the companys purchasing manager and director of finance that the company should invest in a sophisticated new...
-
How do postcolonial authors utilize allusions to colonial histories and indigenous traditions as a means of reclaiming and reinterpreting cultural narratives, subverting colonial perspectives, and...
-
Alliance Companys budgets production of 25,000 units in January and 29,000 units in the February. Each finished unit requires 4 pounds of raw material K that costs $3.50 per pound. Each months ending...
-
Division A has variable manufacturing costs of $53 per unit and fixed costs of $14 per unit. Assuming that Division A is operating significantly below capacity, what is the optimal transfer price of...

Study smarter with the SolutionInn App


