Exactly 1.00 mol each of CO and Cl 2 are introduced into an evacuated 1.75 L flask,
Question:
Exactly 1.00 mol each of CO and Cl2 are introduced into an evacuated 1.75 L flask, and the following equilibrium is established at 668 K.
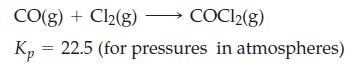
For this equilibrium, calculate
(a) The partial pressure of COCl2(g);
(b) The total gas pressure.
Transcribed Image Text:
CO(g) + Cl₂(g) COC12(g) Kp 22.5 (for pressures in atmospheres)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The partial pressure of COCl2g and the total gas pressure for the given equilibrium a Partial pressu...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
(A) A 5.00 L evacuated flask is filled with 1.86 mol NOBr. At equilibrium at 25 C, there is 0.082 mol of Br 2 present. Determine K c and K p for the reaction 2 NOBr(g) 2 NO(g) + Br 2 (g). (B) 0.100...
-
A mixture of 0.2000 mol of CO2, 0.1000 mol of H2, and 0.1600 mol of H2O is placed in a 2.000-L vessel. The following equilibrium is established at 500 K: (a) Calculate the initial partial pressures...
-
Dr. Bold has a personal automobile policy with liability limits as follows: $100,000/$300,000 BI and $50,000 PD. Dr. Bold is held liable in an accident in which he must pay for bodily injuries as...
-
Data-Check is considering two capital structures. The key information is shown in the following table. Assume a 40% tax rate. a. Calculate two EBIT-EPS coordinates for each of the structures by...
-
Plaintiff, John W. Carson, was the host and star of The Tonight Show, a well-known television program broadcast by the National Broadcasting Company. Carson also appeared as an entertainer in...
-
In 2014, Barker contacted Price about a van Price had advertised for sale. The advertisement described the van as a 1994 Ford E350. Barker and Price agreed to meet, and, on April 9, Barker inspected...
-
Wie Company has been operating for just 2 years, producing specialty golf equipment for women golfers. To date, the company has been able to finance its successful operations with investments from...
-
Research articles on Online Analytic Processing (OLAP) and Online Transaction Processing (OLTP). Next, compare and contrast the key similarities and differences between Online Analytical Processing...
-
For the reaction 2 NO 2 (g) 2 NO(g) + O 2 (g), K c = 1.8 x 10 -6 at 184 C. What is the value of K p for this reaction at 184 C, for pressures expressed in atmospheres? (8)702 + NO(g) + O2(g) NO2(g)
-
Concerning the reaction in Exercise 51, if KO 2 (s) and K 2 CO 3 (s) are maintained in contact with air at 1.00 atm and 25 C, in which direction will a net change occur to establish equilibrium?...
-
The following information is taken from the notes of the statement of financial position (balance sheet) of a listed company. Write a short explanation that is suitable for a private shareholder who...
-
A model airplane is being flown on a guideline that can sustain at most 180 N of tension. The mass of the plane is 0.75 kg, and its speed is 36.1 m/s. Assuming that the guideline is parallel to the...
-
Consider the linear equations x + 2x2 = 4, - 2x1 x = 1. (a) Write the linear system of equations in the matrix form Ax = 6 as we did in class. (b) Use the determinant to show that A is invertible....
-
a. A CNC machine center has a burden rate of $280/hr. Setup time is 1.9 hours. Each part requires 6 minutes to complete? b. If a lot size is 275 parts, how much of the part cost is setup? c. A lot...
-
A) what is f'(2) B) What is the average rate of change in f' between x=2 and x=3? C) what is f"(2)? tip de A 2 Gyor 4 asili y Figure 2.52 f(x) x
-
Ms. Gamboa uses accrual method of accounting. She owns a land which she leased to Mr. Sotto for 2 years at an annual rental of P 100,000. On July 1, 2018, she received P 200,000 from Mr. Sotto...
-
Heckaman Corporation produces and sells a single product. Data concerning that product appear below. Selling price per unit ............... $230.00 Variable expense per unit ........ $112.70 Fixed...
-
Design a circuit which negative the content of any register and store it in the same register.
-
Rejuvenation Physical Therapy Inc. is planning its cash payments for operations for the third quarter (July?September), 2011. The Accrued Expenses Payable balance on July 1 is $24,000. The budgeted...
-
On January 1, 2010, the controller of Gardeneer Tools Inc. is planning capital expenditures for the years 20102013. The following interviews helped the controller collect the necessary information...
-
Guardian Devices Inc. prepared the following sales budget for the current year: At the end of December 2010, the following unit sales data were reported for the year: For the year ending December 31,...
-
5. a 2 -3 M=2 3 0 where a is a constant 4 a 2 (a) Show that M is non-singular for all values of a. (b) Determine, in terms of a, M (2) (4)
-
Use these financial statements to answer the questions. Balance Sheet 2 0 0 6 2 0 0 7 2 0 0 6 2 0 0 7 Cash $ 2 , 4 0 0 $ 3 , 2 0 0 Accounts payable $ 9 , 4 0 0 $ 1 3 , 5 0 0 Accounts receivable 8 , 1...
-
(a) Describe an algorithm that sorts an input array A[1..n] by calling a subroutine SQRTSORT(K), which sorts the subarray A[k+1..k+ n] in place, given an arbitrary integer k between 0 and n-n as...

Study smarter with the SolutionInn App


