Question: For the voltaic cell, (a) what isE cell initially? (b) As the cell operates, willE cell increase, decrease, or remain constant with time? Explain. (c)
For the voltaic cell,
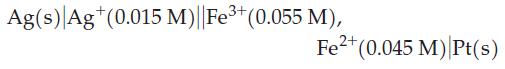
(a) what is Ecell initially?
(b) As the cell operates, will Ecell increase, decrease, or remain constant with time? Explain.
(c) What will be Ecell when [Ag+] has increased to 0.020 M?
(d) What will be [Ag+] when Ecell = 0.010 V?
(e) What are the ion concentrations when Ecell = 0?
Ag(s) Ag+ (0.015 M)||Fe+ (0.055 M), Fe+ (0.045 M) Pt(s)
Step by Step Solution
3.46 Rating (162 Votes )
There are 3 Steps involved in it
a To calculate the initial Ecell we need to use the Nernst equation Ecell Ecell RTnFlnQ where Ecell is the standard cell potential R is the gas consta... View full answer

Get step-by-step solutions from verified subject matter experts


